1405-41-0
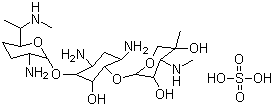
- Product Name:Gentamycin sulfate
- Molecular Formula:C21H43N5O7.H2SO4
- Purity:99%
- Molecular Weight:
Product Details
Appearance:White crystalline powder
Factory sells 1405-41-0 Gentamycin sulfate in stock
- Molecular Formula:C21H43N5O7.H2SO4
- Molecular Weight:575.67
- Appearance/Colour:White crystalline powder
- Melting Point:218-237 °C
- Boiling Point:797.6 °C at 760 mmHg
- Flash Point:436.2 °C
- PSA:719.38000
- Density:==
- LogP:-1.26180
Gentamycin sulfate 1405-41-0 Good Supplier In China
Gentamicin Sulfate is the sulfate salt form of gentamycin, a broad-spectrum aminoglycoside antibiotic complex produced by the fermentation of Micromonospora purpurea or M. echinospora, with antibacterial activity. Gentamicin is a thermostable complex containing the gentamicins C1, C1a, C2, C2a and C2b. It is used to treat serious bacterial infections in many different parts of the body. Gentamicin belongs to the class of medicines known as aminoglycoside antibiotics. Topical gentamicin is used in adults and children 1 year of age and older to treat skin infections caused by certain bacteria. Topical gentamicin is in a class of medications called antibiotics. It works by killing bacteria that cause infections.
Fire Hazard
Flash point data for Gentamycin sulfate are not available; however, Gentamycin sulfate is probably combustible.
InChI:InChI=1/C19H39N5O7.H2O4S/c1-19(27)7-28-18(13(26)16(19)24-2)31-15-11(23)5-10(22)14(12(15)25)30-17-9(21)4-3-8(6-20)29-17;1-5(2,3)4/h8-18,24-27H,3-7,20-23H2,1-2H3;(H2,1,2,3,4)/t8-,9+,10-,11+,12-,13+,14+,15-,16+,17+,18+,19-;/m0./s1
163222-33-1 Gentamycin sulfate Relevant articles
Determination of Gentamycin Sulfate in Gentamycin Sulfate Eye Drops by Rapid Colorimetric Assay
Jianhua Li
《China Modern Doctor》, (2008)
Fading Spectrophotometric Method for the Determination of Gentamycin Sulfate with Azo blue
X Hong
Chemical Industry Times 2007-08
The interaction of Gentamycin Sulfate and Azo blue was studied by fading spectrophotometry.In pH=4.10 Britton Robinson buffer solution,Azo blue had a strong absorbance at 553 nm and the addition of Gentamycin Sulfate into Azo blue solution resulted in the decrease in the absorbance value at 553nm.
In vitro evaluation of the effect of Ceftiofur Sodium and of a new Gentamycin Sulfate formulation on the viability of Marek disease virus
J. Chacon, M. Pimentel, A. Pedroso, A. Ferreira, D. Martinez, C. Ruelas
arXiv preprint arXiv, 2022
The present study evaluated In vitro effect of gentamicin sulfate and ceftiofur sodium on the viability of the Marek's disease virus. The titer of cell associated turkey herpesvirus (HVT) vaccine was not appreciably reduced when incubated with 50 mg/ml of gentamicin sulfate or ceftiofur sodium. Statistic difference was not found between the number of plaqueforming units (PFU) of reconstituted vaccine associated with both antibiotics 0, 15, 30 and 60 minutes after reconstitution of vaccine. The antibiotics did not considerably alter the pH values. There was a significative decrease of the titer of all vaccinal solutions when they were inoculated 30 and 60 minutes after the reconstitution of the vaccine. Nevertheless, these titers are higher than the required titers to protectect against the Marek disease.
Relevant Products
-
Ampicillin
CAS:7177-48-2
-
Ezetimibe
CAS:163222-33-1
-
(4S,5R)-4-Methyl-5-phenyloxazolidin-2-one
CAS:16251-45-9