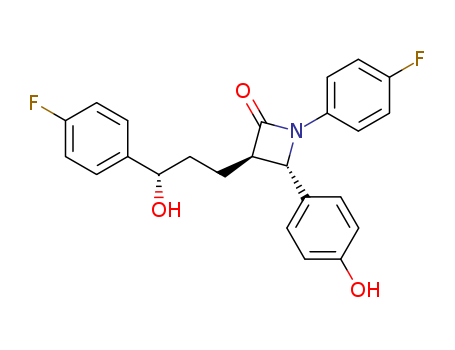
163222-33-1
- Product Name:Ezetimibe
- Molecular Formula:C24H21F2NO3
- Purity:99%
- Molecular Weight:
Product Details
Packing:1g,5g,10g
Appearance:white powder
Wholesaler, low price, Ezetimibe 163222-33-1
Ezetimibe 163222-33-1 is ananti-hyperlipidemic drug used for lowering the plasma cholesterol levels. It is indicated as an adjunctive therapy to diet for the reduction of high-level total-C, LDL-C, and ApoB in patients suffering primary (heterozygous familial and non-familial) hypercholesterolemia.
Ezetimibe 163222-33-1 On Sale
- Molecular Formula:C24H21F2NO3
- Molecular Weight:409.432
- Appearance/Colour:white powder
- Vapor Pressure:4.83E-18mmHg at 25°C
- Melting Point:164-166 °C
- Refractive Index:1.623
- Boiling Point:654.9 °C at 760 mmHg
- PKA:9.72±0.30(Predicted)
- Flash Point:349.9 °C
- PSA:60.77000
- Density:1.334 g/cm3
- LogP:4.95330
Ezetimibe 163222-33-1 Usage
Ezetimibe 163222-33-1 is a once-daily orally active cholesterol absorption inhibitor, launched as a hypolipidemic agent. Ezetimibe appears to be selective in its actions in that it does not interfere with the absorption of triglycerides, lipid-soluble vitamins or other nutrients. For use as adjunctive therapy to diet for the reduction of elevated total-C, LDL-C, and Apo B in patients with primary (heterozygous familial and non-familial) hypercholesterolemia.
Ezetimibe (9) was approved as the first hypolipidemic drug to act by blocking the absorption of dietary cholesterol. This drug was discovered by Schering-Plough and is codeveloped and co-marketed by Merck and Schering-Plough for the treatment of hypercholesterolemia and also two less common forms of hyperlipidemia: homozygous familial hypercholesterolemia and homozygous sitosterolemia. Although it may beused alone, it is marketed as a combination product withsimvastatin under the trade name Vytorin. Ezetimibe is indicated as monotherapy or in combination with an HMGRI for the reduction of elevated total cholesterol, LDL cholesterol, and apoB in patients with primary (heterozygous familial and nonfamilial) hypercholesterolemia. All indications are for patients who have not responded to diet, exercise, and other nonpharmacological methods.
InChI:InChI=1/C24H21F2NO3/c25-17-5-1-15(2-6-17)22(29)14-13-21-23(16-3-11-20(28)12-4-16)27(24(21)30)19-9-7-18(26)8-10-19/h1-12,21-23,28-29H,13-14H2
163222-33-1 Relevant articles
Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study).
-
《American Journal of Cardiology》 CM Ballantyne,R Weiss,T Moccetti,A Vogt,B Eber,F Sosef,E Duffield, (2007)
This study was designed to investigate the efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe 10 mg in patients at high risk of coronary heart disease.
Ezetimibe alone reduces low-density lipoprotein cholesterol in HIV-infected patients receiving combination antiretroviral therapy.
DA Wohl,W David,RJ Simpson,R Susan,S Amanda,N Sonia,K Jessica,EJ Joseph,H Priscilla
, Clinical Infectious Diseases 20081015
In this crossover study of ezetimibe monotherapy in 48 antiretroviral-treated patients with human immunodeficiency virus infection, the mean changes in low-density lipoprotein cholesterol were -5.3% (-11 mg/dL) and + 5.5% (+ 4 mg/dL) with ezetimibe treatment and placebo, respectively (P = .04). Ezetimibe was safe and effective in reducing lowdensity lipoprotein cholesterol and is an option for patients who cannot tolerate treatment with a statin.
Ezetimibe - new anti-atherogenic properties?
-
《British Journal of Pharmacology》 GH Tomkin , (2009/04/01)
Ezetimibe, a Niemann Pick C1-like1 inhibitor, inhibits cholesterol absorption. The drug has been shown to affect lipid raft function in monocytes and therefore may inhibit lipid accumulation in the atheromatous plaque with a mechanism that is unrelated to its effect in reducing cholesterol absorption.
163222-33-1 Process route
-
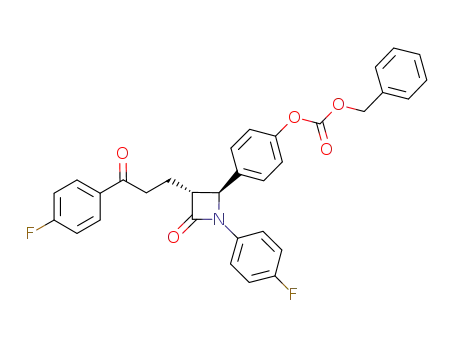
- 1159183-23-9
C32H25F2NO5

-
![[14C]-Sch 57871](/upload/2023/1/9c047a41-1557-434b-b991-fd81557df5a6.png)
- 191330-56-0
[14C]-Sch 57871

-
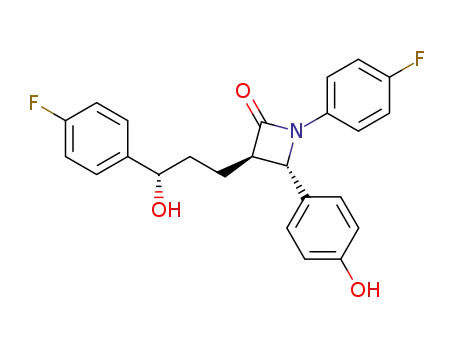
- 163222-33-1,1478664-18-4,1593543-00-0,1593543-07-7,1478664-02-6
ezetemibe
| Conditions | Yield |
|---|---|
|
With D-glucose; at 30 ℃; for 48h; pH=7; diastereoselective reaction; Microbiological reaction; aq. phosphate buffer;
|
-

- 1159183-23-9
C32H25F2NO5

-
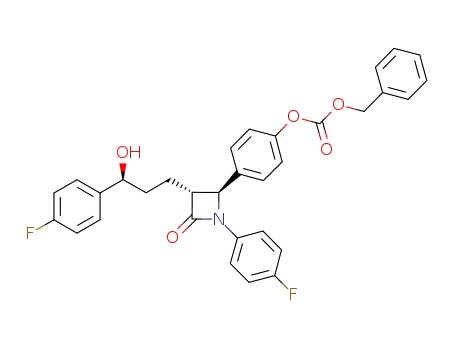
- 1159183-24-0
benzyl (4-((2S,3R)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-oxoazetidin-2-yl)phenyl) carbonate

-
![[14C]-Sch 57871](/upload/2023/1/9c047a41-1557-434b-b991-fd81557df5a6.png)
- 191330-56-0
[14C]-Sch 57871

-

- 163222-33-1,1478664-18-4,1593543-00-0,1593543-07-7,1478664-02-6
ezetemibe
| Conditions | Yield |
|---|---|
|
With D-glucose; at 30 ℃; for 24h; pH=7; diastereoselective reaction; Microbiological reaction; aq. phosphate buffer;
|
163222-33-1 Upstream products
-
163222-32-0
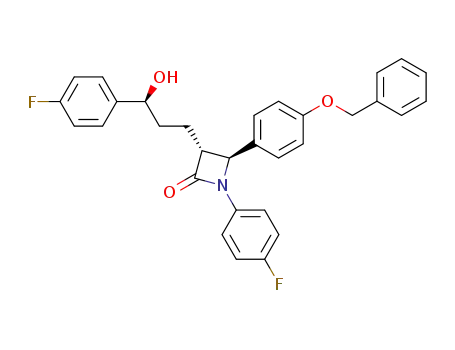
(3R,4S)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4-(4-benzyloxyphenyl)-2-azetidinone
-
191330-56-0
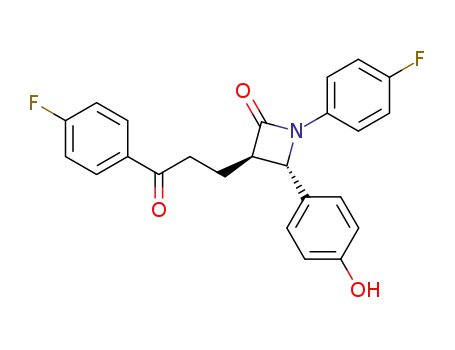
[14C]-Sch 57871
-
954109-26-3
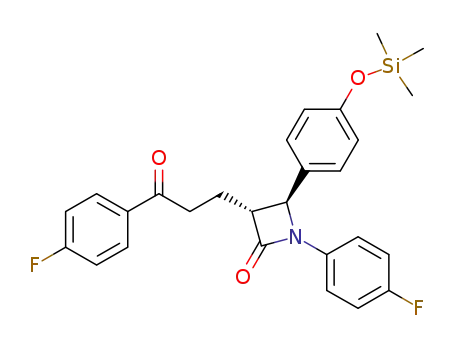
(3R,4S)-4-(4-(trimethylsilyloxy)phenyl)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one
-
190595-65-4
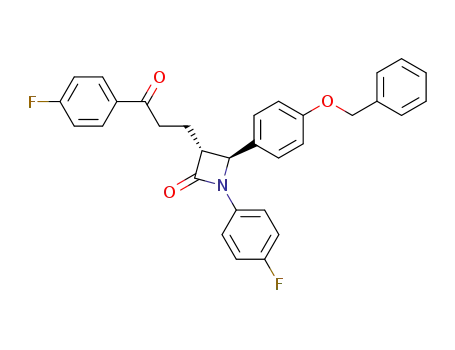
(3R,4S)-4-[4-(benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one
163222-33-1 Downstream products
-
163380-20-9
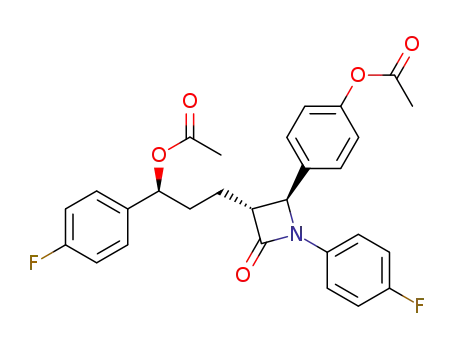
4(S)-(4-acetyloxyphenyl)-3(R)-(3(S)-acetyloxy-3-(4-fluorophenyl)propyl)-1-(4-fluorophenyl)-2-azetidinone
-
795306-53-5
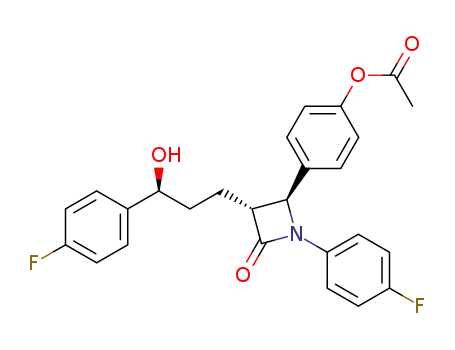
Acetic acid 4-{(2S,3R)-1-(4-fluoro-phenyl)-3-[(S)-3-(4-fluoro-phenyl)-3-hydroxy-propyl]-4-oxo-azetidin-2-yl}-phenyl ester
-
847781-45-7
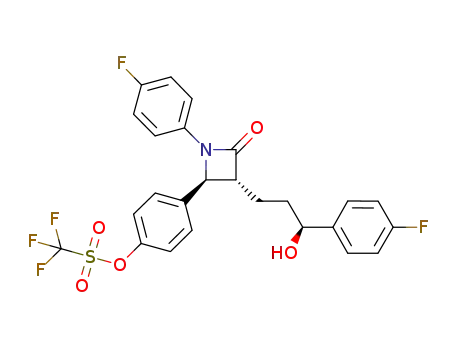
(trifluoromethanesulfonyl)ezetimibe
-
847694-38-6
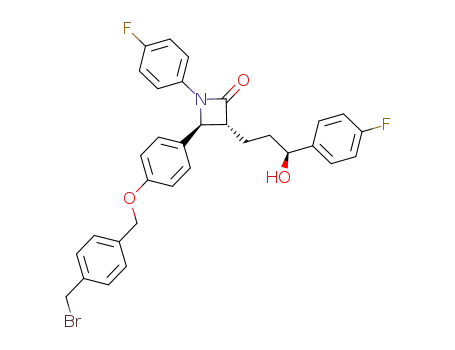
(3R,4S)-4-[4-(4-bromomethylbenzyloxy)phenyl]-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]azetidin-2-one
Relevant Products
-
High quality Ticagrelor 99%
CAS:274693-27-5
-
N-(tert-Butoxycarbonyl)-4-piperidone
CAS:79099-07-3
-
Gentamycin sulfate
CAS:1405-41-0