7177-48-2
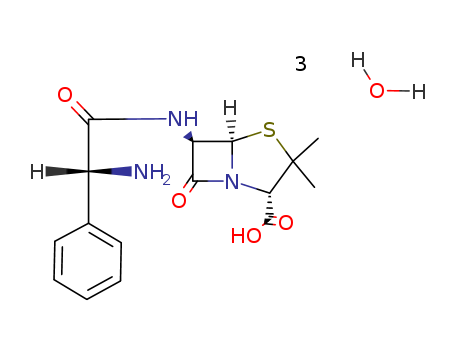
- Product Name:Ampicillin
- Molecular Formula:C16H19N3O4S.3(H2O)
- Purity:99%
- Molecular Weight:
Product Details
Appearance:Odorless white microcrystalline powder with a bitter taste
7177-48-2 superior manufacturer, factory sells Ampicillin
- Molecular Formula:C16H19N3O4S*3H2O
- Molecular Weight:403.456
- Appearance/Colour:Odorless white microcrystalline powder with a bitter taste
- Vapor Pressure:1.21E-19mmHg at 25°C
- Melting Point:208 °C (dec.)(lit.)
- Refractive Index:265 ° (C=0.1, H2O)
- Boiling Point:683.9 °C at 760 mmHg
- PKA:2.5 (COOH)(at 25℃)
- Flash Point:367.4 °C
- PSA:165.72000
- LogP:1.15430
7177-48-2 Usage
Ampicillin (AMPI) is a commonly used antibiotic for treating pathogenic bacteria and is additionally added to livestock feed in agriculture. Ampicillin is the first broad-spectrum penicillin, which has in vitro activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria, commonly used for preventing and treating bacterial infections of respiratory tract, bladder, and kidney, etc. caused by susceptible bacteria.
Safety Profile
Mildly toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of SO,xand NOx.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
InChI:InChI=1/C16H19N3O4S.3H2O/c1-16(2)11(15(22)23)19-13(21)10(14(19)24-16)18-12(20)9(17)8-6-4-3-5-7-8;;;/h3-7,9-11,14H,17H2,1-2H3,(H,18,20)(H,22,23);3*1H2/t9-,10-,11+,14-;;;/m1.../s1
7177-48-2 Relevant articles
Capability of MXene 2D material as an amoxicillin, ampicillin, and cloxacillin adsorbent in wastewater
Ahmad Miri-Jahromi, Mohsen Didandeh, Sina Shekarsokhan
, Journal of Molecular Liquids Volume 351, 1 April 2022, 118545
Amoxicillin, ampicillin, and cloxacillin are three semi-synthetic antibiotic compounds that pollute wastewaters.
A CRISPR/Cas12a-based fluorescence aptasensor for the rapid and sensitive detection of ampicillin
Bong Jing Yee a, Nurul Faizeemah Shafiqah a, Noor Faizah Mohd-Naim b, Minhaz Uddin Ahmed a
International Journal of Biological Macromolecules Volume 242, Part 4, 1 July 2023, 125211
In this study, ampicillin was successfully detected with a rapid aptasensor based on the CRISPR/Cas12a system. The fluorescence signal was produced from the trans-cleavage of ssDNA reporter probes when the Cas12a was activated by the target ssDNA sequence upon aptamer interaction with AMPI. The fluorescence signal response showed a strong linear relationship with the concentration AMPI at as low as 0.01 nM.
Degradation of ampicillin by combined process: Adsorption and Fenton reaction
Cassandra Bonfante de Carvalho, Ivan Reis Rosa, Paola Del Vecchio, Ivone Vanessa Jurado Dávila, Keila Guerra Pacheco Nunes, Nilson Romeu Marcilio, Liliana Amaral Féris
Environmental Technology & Innovation Volume 26, May 2022, 102365
In this context, the present work investigated the degradation and mineralization of the -lactam antibiotic ampicillin (AMP) applying a combined treatment of Fenton reaction and adsorption onto granular activated carbon (GAC).
7177-48-2 Upstream products
-
551-16-6
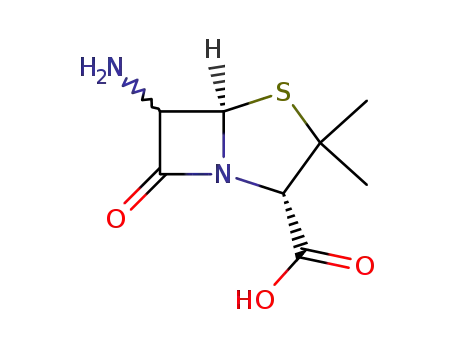
6-aminopenicillanic acid
-
5321-31-3
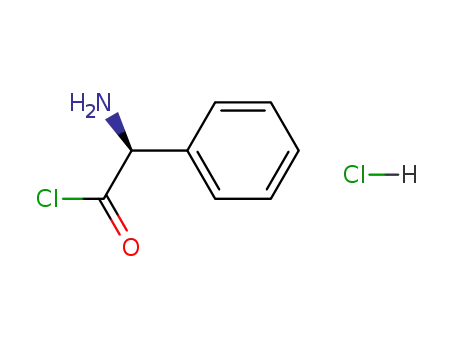
phenylglycidylchloride hydrochloride
-
551-16-6
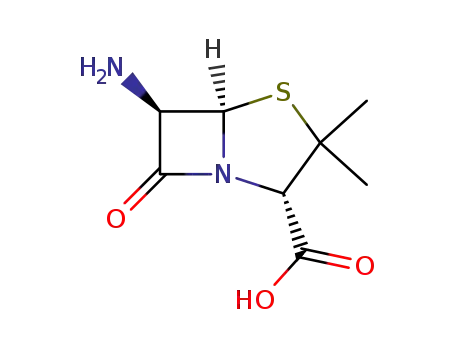
6-Aminopenicillanic Acid
-
18297-63-7
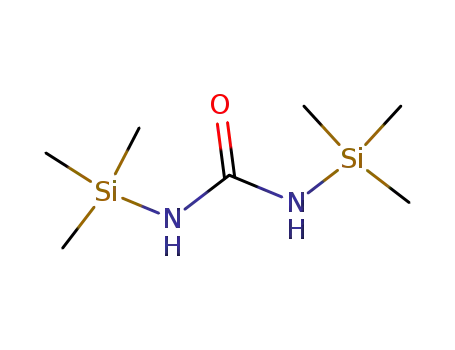
N,N'-bis(trimethylsilyl)urea
7177-48-2 Downstream products
-
87-53-6
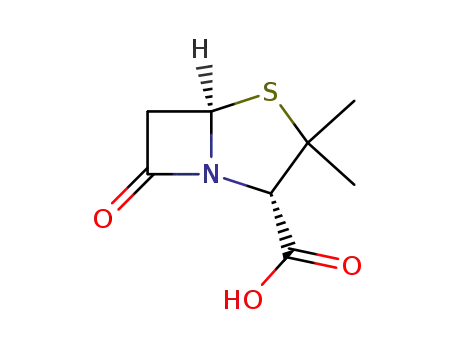
penicillanic acid
-
61-33-6
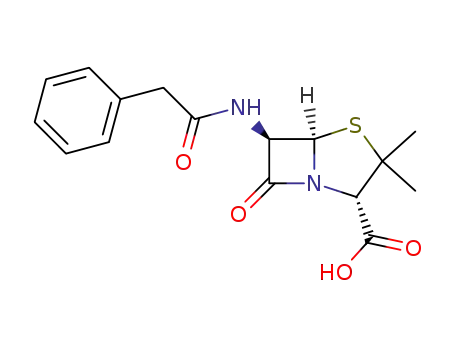
penicillin G
Relevant Products
-
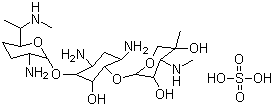
Gentamycin sulfate
CAS:1405-41-0
-
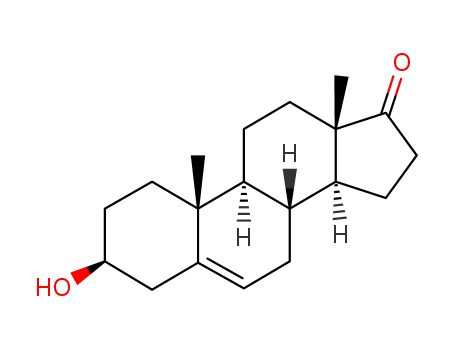
Dehydroepiandrosterone
CAS:53-43-0
-
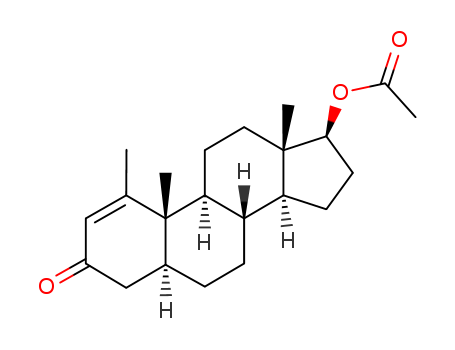
Methenolone Acetate
CAS:434-05-9