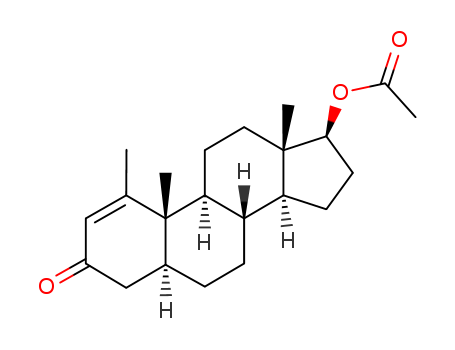
434-05-9
- Product Name:Methenolone Acetate
- Molecular Formula:C22H32O3
- Purity:99%
- Molecular Weight:
Product Details
Methenolone Acetate 434-05-9 supplier, in stock, low price
- Molecular Formula:C22H32O3
- Molecular Weight:344.494
- Vapor Pressure:5.54E-08mmHg at 25°C
- Melting Point:138-139°
- Refractive Index:1.536
- Boiling Point:441.2 °C at 760 mmHg
- Flash Point:189.9 °C
- PSA:43.37000
- Density:1.1 g/cm3
- LogP:4.69600
434-05-9 Usage
Methenolone acetate is a synthetic anabolic-androgenic steroid that acts as a potent androgen and has been marketed as a treatment for inoperable breast cancer. It can be used for the treatment of bone marrow disease and anemia. Metenolone acetate is a long-acting anabolic steroid with weak androgenic properties. It is capable of enhancing performance in racehorses. Methenolone acetate is a synthetic anabolic steroid and hence an agonist of the androgen receptor, which is the biological target of androgens such as testosterone and dihydrotestosterone, that is still used in Japan in the treatment of anemia due to bone marrow failure, unlike in Europe and the United States, where it is no longer used for this purpose. Moreover, this drug has been approved in Japan for more than 50 years for the treatment of osteoporosis.
InChI:InChI=1/C22H32O3/c1-13-11-16(24)12-15-5-6-17-18-7-8-20(25-14(2)23)21(18,3)10-9-19(17)22(13,15)4/h11,15,17-20H,5-10,12H2,1-4H3/t15-,17-,18-,19-,20-,21-,22-/m0/s1
434-05-9 Relevant articles
Development and validation of RP-HPLC method for analytical characterization of the anabolic steroid Methenolone acetate in food supplements
Diana Tzankova , Alexandrina Mateeva , Javor Mitkov , Lily Peikova , Maya Georgieva
, Pharmacia 69(1): 151–155
A necessity of the health risk associated with their unregu-lated usage requires development of suitable fast and precise methods for their evaluation. The obtained result lead us to the conclusion that the identified in the label Methenolone acetate is found as im-purity rather than as a main constituent.
LC-MS/(MS) confirmatory doping control analysis of intact phase II metabolites of methenolone and mesterolone after Girard's Reagent T derivatization
Yiannis S. Angelis, Argyro G. Fragkaki, Polyxeni Kiousi, Panagiotis Sakellariou, Christophoros Christophoridis
, Drug Testing and Analysis 2023
For the evaluation of the proposed protocols, urine samples from methenolone and mesterolone excretion studies were analyzed against at least one sample from a different excretion study.
Attenuation of Bone Mineral Density Decline During Anemia Treatment With Methenolone Acetate in Myelodysplastic Syndrome
Shu Ushimaru, Hirofumi Sumi, Mea Aso, Rie Fujishima, Kazuhiro Shiizaki, Naoto Tominaga
JCEM Case Reports, Volume 2, Issue 4, April 2024, luae055,
In this case report, we present an older man with stage 4 chronic kidney disease complicated by myelodysplastic syndrome and progressive decline in bone mineral density. He was treated with methenolone acetate and darbepoetin for anemia caused by myelodysplastic syndrome. During anemia treatment, the decline in bone mineral density was attenuated overtime. The case findings suggest the potential association between the use of methenolone acetate as a synthetic anabolic steroid and attenuated decline in bone mineral density.
434-05-9 Upstream products
-
1099-80-5
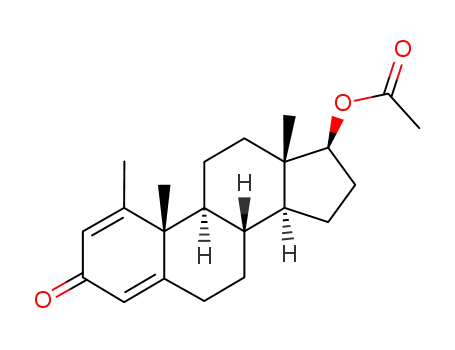
17β-acetoxy-1-methyl-androsta-1,4-dien-3-one
434-05-9 Downstream products
-
127557-42-0
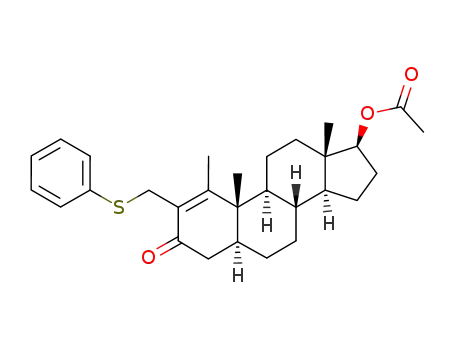
17β-acetyloxy-1-methyl-2-<(phenylthio)methyl>-5α-androst-1-en-3-one
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
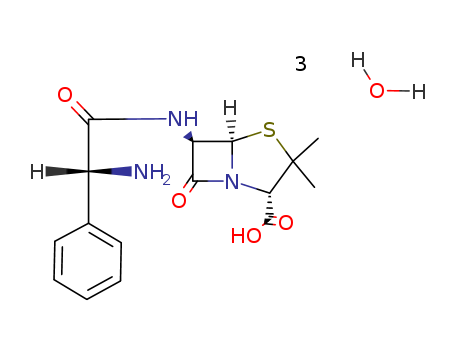
Ampicillin
CAS:7177-48-2
-
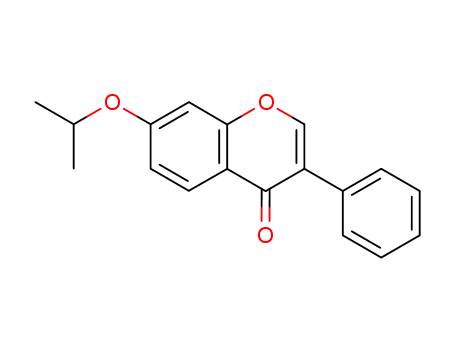
Ipriflavone
CAS:35212-22-7