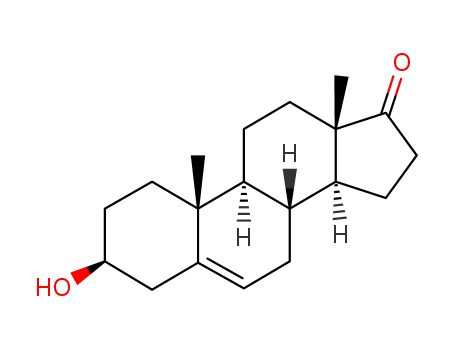
53-43-0
- Product Name:Dehydroepiandrosterone
- Molecular Formula:C19H28O2
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white fine crystalline powder
Best price 53-43-0, factory sells Dehydroepiandrosterone with low price
- Molecular Formula:C19H28O2
- Molecular Weight:288.43
- Appearance/Colour:white fine crystalline powder
- Vapor Pressure:4.54E-09mmHg at 25°C
- Melting Point:149-151 °C(lit.)
- Refractive Index:1.56
- Boiling Point:426.7 °C at 760 mmHg
- PKA:15.02±0.60(Predicted)
- Flash Point:182.1 °C
- PSA:37.30000
- Density:1.12 g/cm3
- LogP:3.87920
Dehydroepiandrosterone 53-43-0 Usage
Dehydroepiandrosterone (DHEA) is a steroid hormone that is a popular nonprescription oral “dietary supplement” used by men to enhance cognitive function, mood, libido, and athletic performance. Dehydroepiandrosterone (DHEA) is an androgen produced by the zona reticularis of the adrenal gland. The biological significance of dehydroepiandrosterone (DHEA) which, in the form of its sulfated ester is the most abundant steroid hormone in human plasma, is an enigma.
InChI:InChI=1/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h3,13-16,20H,4-11H2,1-2H3/t13-,14-,15?,16?,18-,19-/m0/s1
53-43-0 Relevant articles
Functions of dehydroepiandrosterone in relation to breast cancer
Robert T. Chatterton
Steroids Volume 179, March 2022, 108970
Dehydroepiandrosterone sulfate (DS) and DHEA have an association with increasing lifespan, yet there is a declining benefit as people age.
A steroidogenic pathway for sulfonated steroids: The metabolism of pregnenolone sulfate
Neunzig,Sánchez-Guijo,Mosa,Hartmann,Geyer,Wudy,Bernhardt
, p. 324 - 333 (2014)
In many tissues sulfonated steroids exceed the concentration of free steroids and recently they were also shown to fulfill important physiological functions. We could confirm that PregS is metabolized to 17OH-PregS, strengthening the potential physiological meaning of a pathway for sulfonated steroids.
Dehydroepiandrosterone sulfate (DS) and DHEA have an association with increasing lifespan, yet there is a declining benefit as people age.
Asmaa A. Mahmoud, Asmaa M. Elfiky, Faten S. Abo-Zeid
Steroids Volume 177, January 2022, 108936
Dehydroepiandrosterone (DHEA) administration to prepubertal rats stimulates androgen biosynthesis and generation of the PCOS model. The present study aimed to evaluate the anti-androgenic effects of quercetin (Q) in comparison with metformin (MET) on hyperandrogenism and ovarian dysfunction in a DHEA-induced PCOS rat model.
53-43-0 Process route

-
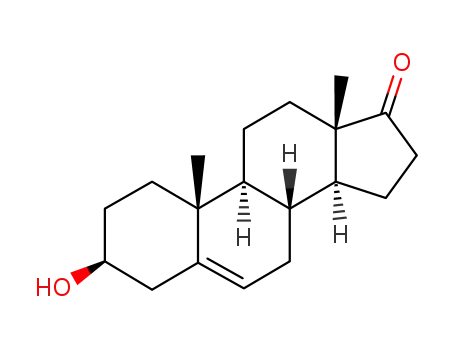
- 53-43-0
dehydroepiandrosterone

-
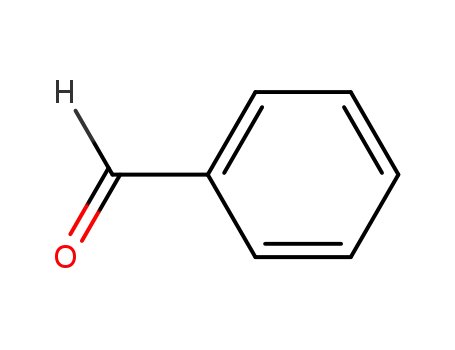
- 100-52-7
benzaldehyde
| Conditions | Yield |
|---|---|
|
|
-
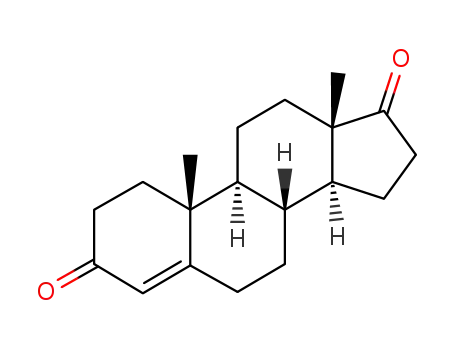
- 63-05-8
Androstenedione

-

- 53-43-0
dehydroepiandrosterone
| Conditions | Yield |
|---|---|
|
Androstenedione; With titanium(IV) oxide; platinum; In toluene; at 100 ℃; for 3h;
With hydrogen; In toluene; for 8h; under 15001.5 Torr; Temperature; Reagent/catalyst; Pressure;
|
85% |
|
Multi-step reaction with 2 steps
1.1: potassium tert-butylate / tert-butyl alcohol / 1.5 h / 35 - 40 °C / Inert atmosphere
1.2: 20 - 25 °C
2.1: NADP; magnesium(II) chloride hexahydrate; alpha-D-glucopyranose; NAD+ / 2-methyltetrahydrofuran; aq. phosphate buffer / 20 - 32 °C / Enzymatic reaction
With alpha-D-glucopyranose; magnesium(II) chloride hexahydrate; potassium tert-butylate; NADP; NAD+; In 2-methyltetrahydrofuran; aq. phosphate buffer; tert-butyl alcohol;
|
|
|
Multi-step reaction with 2 steps
1: potassium tert-butylate / tert-butyl alcohol / 1 h / 30 - 35 °C / Inert atmosphere
2: D-glucose; NAD; glucose dehydrogenase (CDX-901)_; ketoreductase from Sphingomonas wittichii; potassium carbonate / ethyl acetate; aq. phosphate buffer / 21 h / 32.5 °C / pH 6.3 - 6.5 / Inert atmosphere; Enzymatic reaction
With D-glucose; glucose dehydrogenase (CDX-901)_; ketoreductase from Sphingomonas wittichii; potassium tert-butylate; NAD; potassium carbonate; In aq. phosphate buffer; ethyl acetate; tert-butyl alcohol;
|
|
|
Multi-step reaction with 4 steps
1: aluminum (III) chloride; 5-sulfosalicylic Acid / 5 h / 20 - 30 °C / Inert atmosphere; Large scale
2: orthoformic acid triethyl ester; boron trifluoride diethyl etherate / dichloromethane / 5 h / 20 - 30 °C / Inert atmosphere
3: calcium borohydride / dichloromethane / 15 h / -20 - -15 °C
4: toluene-4-sulfonic acid / acetone; water / 1 h / 45 - 50 °C / Inert atmosphere
With aluminum (III) chloride; calcium borohydride; boron trifluoride diethyl etherate; toluene-4-sulfonic acid; orthoformic acid triethyl ester; 5-sulfosalicylic Acid; In dichloromethane; water; acetone;
|
53-43-0 Upstream products
-
57-88-5
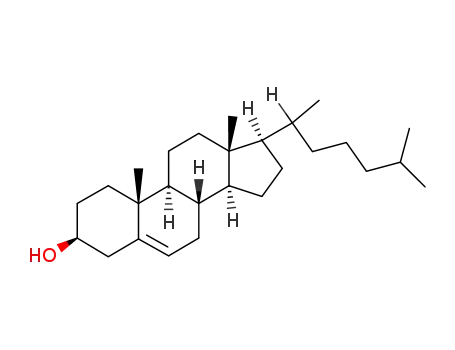
cholesterol
-
83-46-5
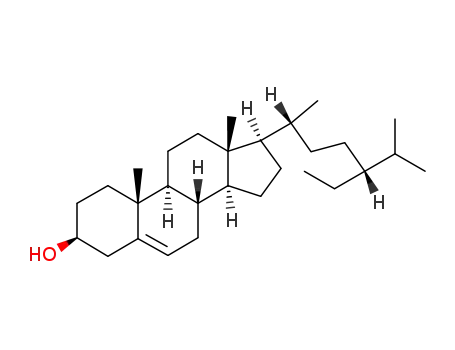
β-sitosterol
-
651-48-9
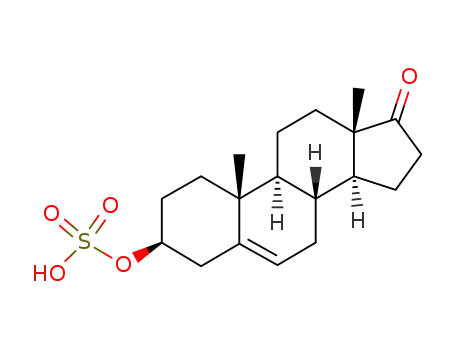
dehydroepiandrosterone sulfate
-
571-36-8
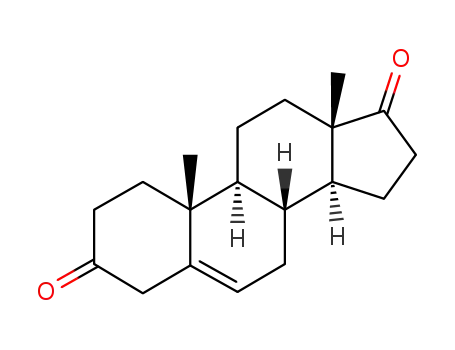
5-androstenedione
53-43-0 Downstream products
-
5419-51-2
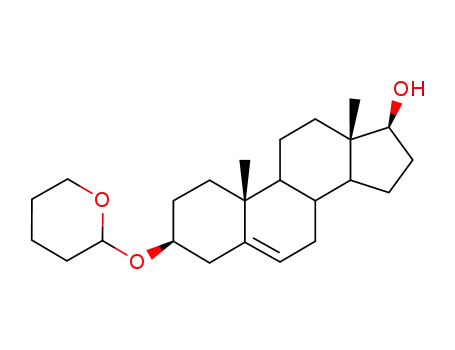
3β-(2-tetrahydropyranyloxy)-5-androstene-17β-0l
-
628-13-7
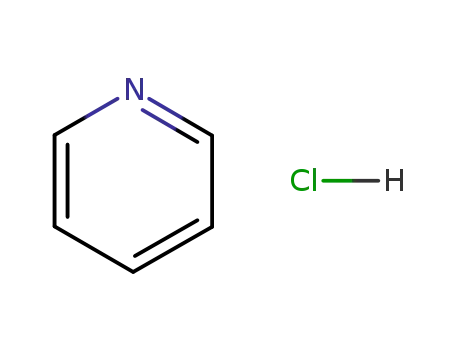
pyridine hydrochloride
-
521-17-5
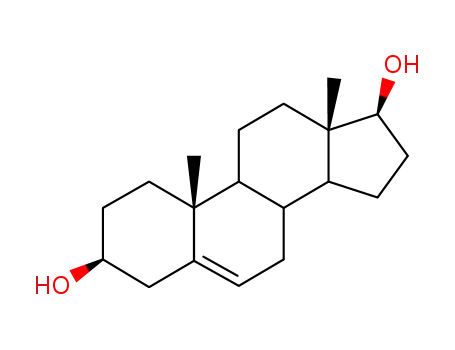
androst-5-ene-3β,17β-diol
-
63015-10-1
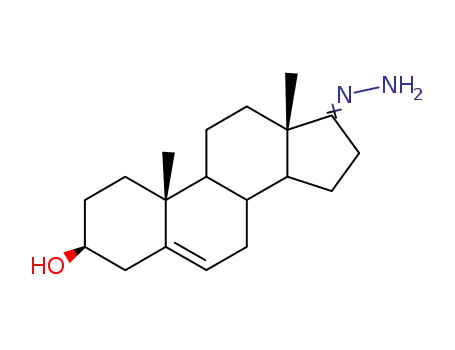
3β-Hydroxy-17-hydrazono-androsten-(5)
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
Methyltetrahydrophthalic anhydride
CAS:11070-44-3
-
Ampicillin
CAS:7177-48-2