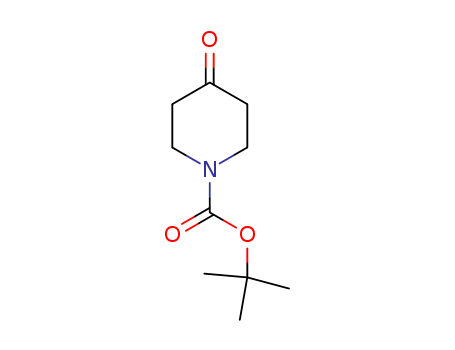
79099-07-3
- Product Name:N-(tert-Butoxycarbonyl)-4-piperidone
- Molecular Formula:C10H17NO3
- Purity:99%
- Molecular Weight:
Product Details
Appearance:light yellow powder
Buy 79099-07-3 N-(tert-Butoxycarbonyl)-4-piperidone in stock with low price.
N-(tert-Butoxycarbonyl)-4-piperidone is used as a pharmaceutical intermediate. We provide N-(tert-Butoxycarbonyl)-4-piperidone with low price.
Hot Sale, low price N-(tert-Butoxycarbonyl)-4-piperidone On Sale
- Molecular Formula:C10H17NO3
- Molecular Weight:199.25
- Appearance/Colour:light yellow powder
- Vapor Pressure:0.00215mmHg at 25°C
- Melting Point:73-77 ºC
- Refractive Index:1.481
- Boiling Point:289.8 ºC at 760 mmHg
- PKA:-1.58±0.20(Predicted)
- Flash Point:129.1 ºC
- PSA:46.61000
- Density:1.099 g/cm3
- LogP:1.52430
79099-07-3 Usage
N-(tert-Butoxycarbonyl)-4-piperidone is also known as N-boc-4-piperidone, is a chemical intermediate that belongs to the group of p2. It is an ancillary used as a pharmaceutical intermediate. It is involved in the preparation of 1-piperidin-4-yl-substituted butyro- and valerolactams. This drug has been shown to inhibit the growth of skin cancer cells and may also be useful in treating other types of cancer, and the preparation of a novel spirocyclic template from tert-butoxycarbonyl-4-piperidone is reported.
InChI:InChI=1/C10H17NO3/c1-10(2,3)14-9(13)11-6-4-8(12)5-7-11/h4-7H2,1-3H3
79099-07-3 Relevant articles
Preparation of 3-Pyrrolidone and 4-Perhydroazepinone
A. Roglans,J. Marquet &M. Moreno-Mañas
, Synthetic Communications Volume 22, 1992 - Issue 9
The sixmembered ring of N-tertbutoxycarbonyl-4-piperidone, 14,6 was expanded. Efficient multigram preparations of 3-pyrrolidone by sequential Michael addition and Dieckmann condensation, and of 4-perhydroazepinone by ring expansion have been achieved.
ANTI-CD25 ANTIBODY-MAYTANSINE CONJUGATES AND METHODS OF USE THEREOF
-
Paragraph 00402-00405, (2021/04/10)
The present disclosure provides anti-CD25 antibody-maytansine conjugate structures. The disclosure also encompasses methods of production of such conjugates, as well as methods of using the same.
ANTI-CD37 ANTIBODY-MAYTANSINE CONJUGATES AND METHODS OF USE THEREOF
-
Paragraph 00409-00412, (2021/05/07)
The present disclosure provides anti-CD37 antibody-maytansine conjugate structures. The disclosure also encompasses methods of production of such conjugates, as well as methods of using the same.
tert-Butyl 3-[N-(tert-butoxycarbonyl) methylamino]-4-methoxyimino-3-methylpiperidine-1-carboxylate
Z Wan, Y Chai, M Liu, H Guo
Crystallographic Communications
The title compound, C18H33N3O5, was prepared from N-tert-butoxycarbonyl-4-piperidone using a nine-step reaction, including condensation, methylation, oximation, hydrolysis, esterification, ammonolysis, Hoffmann degradation, tert-butoxycarbonyl protection and methylation. The E configuration of the methyloxime geometry of the compound is confirmed.
79099-07-3 Process route
-
- 29110-74-5
3-chloroindazole

-
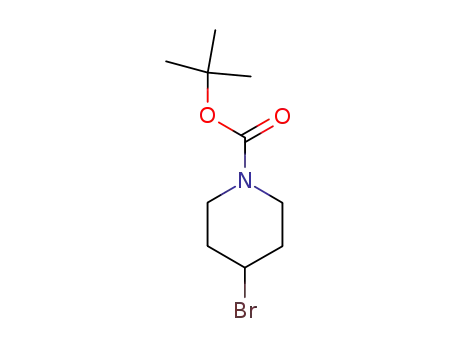
- 180695-79-8
tert-butyl 4-bromo-1-piperidinecarboxylate

-
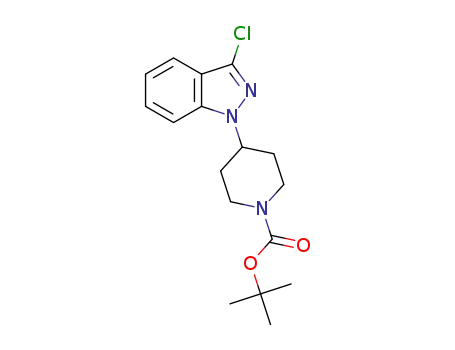
-
C17H22ClN3O2

-
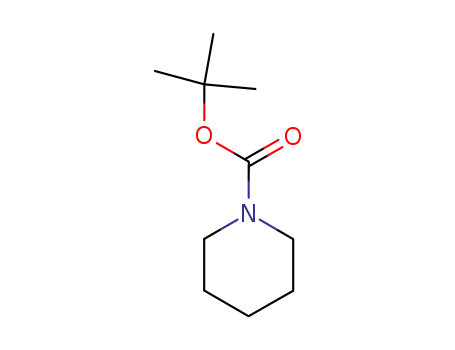
- 75844-69-8
t-butyl piperidinecarboxylate

-
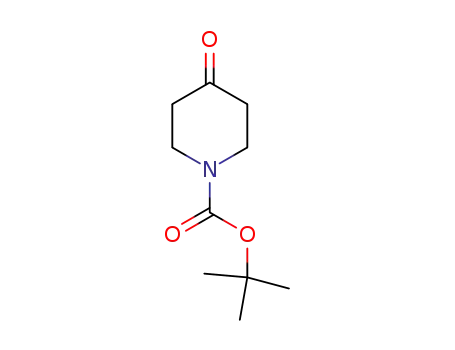
- 79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
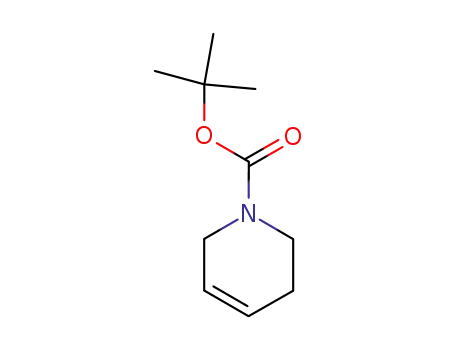
- 85838-94-4
N-Boc-1,2,3,6-tetrahydropyridine
| Conditions | Yield |
|---|---|
|
With copper acetylacetonate; 1,1,1,3,3,3-hexamethyl-2-(trimethylsilyl)-2-trisilanol; [Ir(3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl)2(4,4'-bis(trifluoromethyl)bipyridine)]PF6; N,N,N',N'-tetramethylguanidine; In acetonitrile; at 20 ℃; for 18h; regioselective reaction; Irradiation; Sealed tube;
|
23% |
-
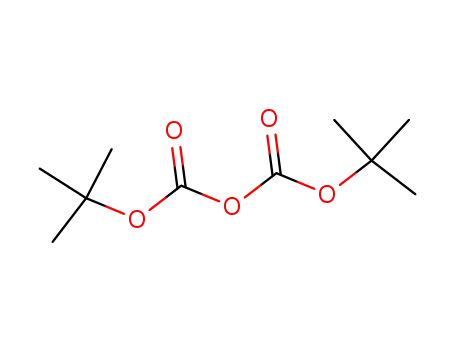
- 24424-99-5
di-tert-butyl dicarbonate

-
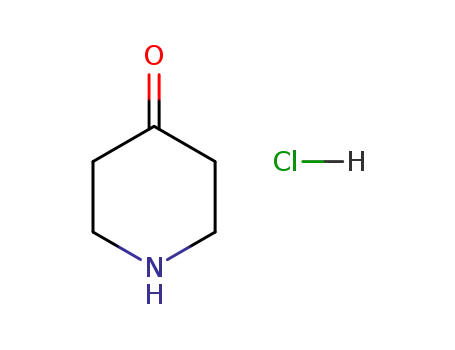
- 41979-39-9
4-piperidone hydrochloride

-

- 79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In water; 1) 35 deg C, 1 h; 2) 50 deg C, 2.5 h;
|
100% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 16h;
|
100% |
|
di-tert-butyl dicarbonate; 4-piperidone hydrochloride; With triethylamine; dmap; In methanol; at 20 ℃; for 20h;
With hydrogenchloride; In dichloromethane;
|
100% |
|
In 1,4-dioxane; water; at 20 ℃; for 4h;
|
100% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 25 ℃; for 6h;
|
98% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 24h;
|
98% |
|
With triethylamine; In 1,4-dioxane; water; at 0 - 20 ℃; Concentration;
|
95% |
|
With sodium hydrogencarbonate; In water; acetone; at 20 ℃; for 24h;
|
94% |
|
With sodium hydrogencarbonate; In 1,4-dioxane; water; at 20 - 70 ℃;
|
93% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 2h; Inert atmosphere;
|
93% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 16h;
|
93% |
|
With sodium hydroxide; In 1,4-dioxane; water; at 0 ℃; for 0.5h;
|
92.5% |
|
With triethylamine; In dichloromethane; at 0 - 20 ℃; for 0.8h;
|
92% |
|
4-piperidone hydrochloride; With triethylamine; In tetrahydrofuran; for 0.0833333h;
di-tert-butyl dicarbonate; With dmap; In tetrahydrofuran; at 20 ℃; for 12h;
|
91.8% |
|
With triethylamine; In N,N-dimethyl-formamide; for 24h; Ambient temperature;
|
90% |
|
4-piperidone hydrochloride; With sodium hydroxide; In water; at 20 - 30 ℃; for 0.333333h;
di-tert-butyl dicarbonate; In water; at 20 - 30 ℃; for 12h; Concentration; Reagent/catalyst;
|
90% |
|
With 1,4-dioxane; triethylamine; In water; at 20 ℃;
|
89% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 16h;
|
88% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 0 - 25 ℃;
|
87.44% |
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 1h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 1h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 1h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; water; at 20 ℃; for 1h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; at 20 ℃;
|
85% |
|
With sodium carbonate; In water; at 35 - 50 ℃; for 4h;
|
81% |
|
With N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; water; at 20 ℃; for 24h;
|
80.2% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 16h;
|
79% |
|
4-piperidone hydrochloride; With triethylamine; In dichloromethane; at 20 ℃; for 0.5h;
di-tert-butyl dicarbonate; In dichloromethane; at 20 ℃; for 16h;
|
79% |
|
4-piperidone hydrochloride; With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20 ℃;
di-tert-butyl dicarbonate; In tetrahydrofuran; water; at 20 ℃; for 12h;
|
78% |
|
With triethylamine; In methanol; dichloromethane; at 0 - 20 ℃; for 16h;
|
78% |
|
With N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; water; at 20 ℃; for 24h;
|
75% |
|
With N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; at 20 ℃; for 24h;
|
75% |
|
With triethylamine; In methanol; at 20 ℃; for 2h;
|
75% |
|
With triethylamine; In tetrahydrofuran; water; for 2h; Ambient temperature;
|
74.4% |
|
With N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; water; at 20 ℃;
|
73.7% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 10 - 20 ℃;
|
65% |
|
With triethylamine; In N,N-dimethyl-formamide; for 18h; Ambient temperature;
|
63% |
|
With sodium hydrogencarbonate; In water; acetonitrile; at 20 ℃;
|
62% |
|
With sodium hydroxide; In water; at 20 ℃; for 6h;
|
40% |
|
With sodium hydroxide; In tetrahydrofuran; at 20 ℃; for 12h;
|
10.9 g |
|
With sodium hydrogencarbonate; sodium chloride; In tetrahydrofuran; water; at 20 ℃;
|
|
|
With sodium hydroxide; In 1,4-dioxane; water; at 20 ℃;
|
|
|
With potassium carbonate; triethylamine; In chloroform; at 0 - 20 ℃;
|
|
|
With sodium hydrogencarbonate; sodium chloride; In tetrahydrofuran; water; at 20 ℃;
|
|
|
With triethylamine; In methanol; at 20 ℃; for 2h;
|
|
|
4-piperidone hydrochloride; With triethylamine; In dichloromethane; for 0.0833333h;
di-tert-butyl dicarbonate; With dmap; In dichloromethane; at 25 ℃; for 20h;
|
3.9 g |
|
With sodium hydroxide;
|
|
|
With sodium hydroxide; sodium monohydrogen sulfate; In tetrahydrofuran;
|
|
|
With triethylamine; In dichloromethane; at 10 - 30 ℃; for 16.25h;
|
79099-07-3 Upstream products
-
41661-47-6
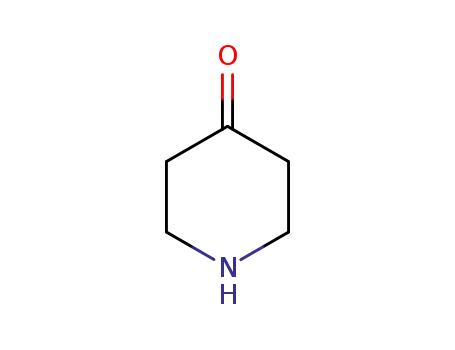
piperidin-4-one
-
24424-99-5

di-tert-butyl dicarbonate
-
41979-39-9

4-piperidone hydrochloride
-
40064-34-4
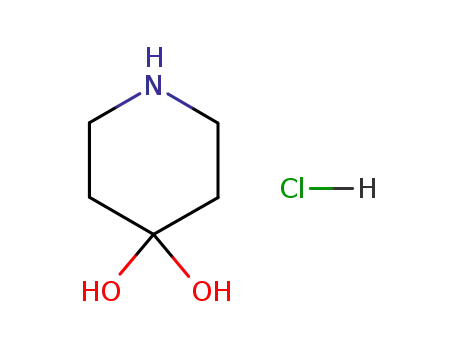
piperidine-4,4-diol hydrochloride
79099-07-3 Downstream products
-
125541-12-0
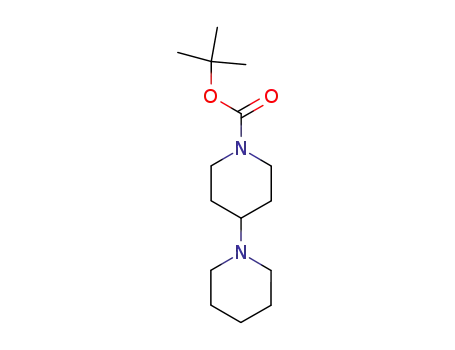
tert-butyl 1,4’-bipiperidine-1’-carboxylate
-
403806-35-9
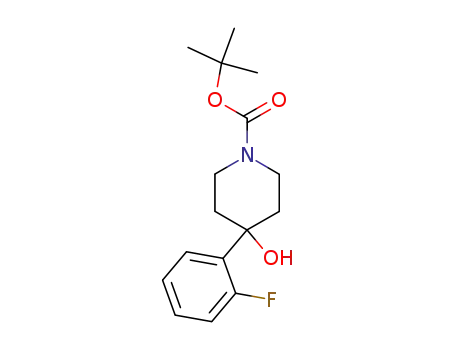
4-(2-fluoro-phenyl)-4-hydroxypiperidine-1-carboxylic acid tert-butyl ester
-
235109-63-4
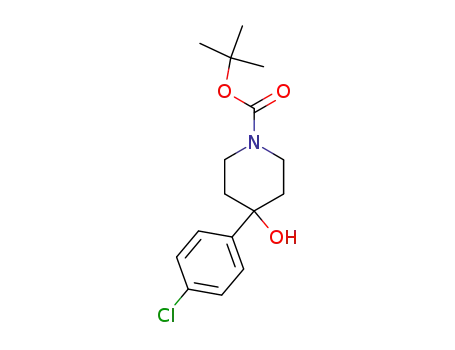
4-(4-chloro-phenyl)-4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester
-
302924-67-0
tert-butyl 4-hydroxy-4-(4-methoxyphenyl)-1-piperidinecarboxylate
Relevant Products
-
Cyclopropane, isocyanato- 4747-72-2
CAS:4747-72-2
-
Calcium propionate, calcium salt (2:1)
CAS:4075-81-4
-
Ezetimibe
CAS:163222-33-1