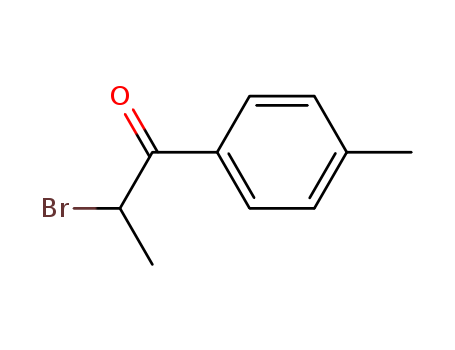
1451-82-7
- Product Name:2-Bromo-4'-Methylpropiophenone
- Molecular Formula:C10H11BrO
- Purity:99%
- Molecular Weight:
Product Details
Appearance:Off-white solid
2-Bromo-4′-methylpropiophenone is usually used as the intermediate and raw material in the fine chemistry industry. Shanghai Upbio Tech Co.,Ltd (Former Onchem (China)Co.,Ltd) is a comprehensive manufacturer and an international distribution of chemicals throughout the world, The predecessor of Shanghai Upbio Tech Co.,Ltd was Onchem (China) Co.,Ltd in 2010,Specialized in APIs, chemical intermediates, herbal extract and pharmaceutical raw materials. We are seeking for extensive cooperation with honest customers.
2-Bromo-4'-Methylpropiophenone superior manufacturer, Factory sells 1451-82-7
- Molecular Formula:C10H11BrO
- Molecular Weight:227.101
- Appearance/Colour:Off-white solid
- Melting Point:75-77 °C
- Boiling Point:273.205 °C at 760 mmHg
- Flash Point:58.628 °C
- PSA:17.07000
- Density:1.357 g/cm3
- LogP:2.96110
1451-82-7 Usage
2-Bromo-4'-methylpropiophenone is an analytical reference standard categorized as a precursor in the synthesis of cathinones. 2-Bromo-4'-methylpropiophenone (cas# 1451-82-7) belongs to the group of phenols, is a compound useful in organic synthesis. It employed as an important intermediate for raw material for organic synthesis, agrochemical, pharmaceutical and dyestuff field. Also used as intermediate for 4-methylmethcathinone.
1451-82-7 Relevant articles
Design, synthesis and broad-spectrum Bcr-Abl inhibitory activity of novel thiazolamide-benzamide derivatives
Liu, Juan,Huang, Honglin,Deng, Xiangping,Xiong, Runde,Cao, Xuan,Tang, Guotao,Wu, Xin,Xu, Shiyu,Peng, Junmei
, p. 2092 - 2101 (2019)
Bcr-Abl plays an important role in the pathogenesis and development of chronic myeloid leukemia (CML). The most potent compound 3m exhibited an Abl IC50 value as low as 1.273 μM and showed inhibition to the T315I mutant with IC50 value 39.89 μM. 3m could prove to be a new promising lead compound for the further development of broad-spectrum Bcr-Abl inhibitors to overcome clinical acquired resistance.
Enantioselective Synthesis of Nitrogen-Nitrogen Biaryl Atropisomers via Copper-Catalyzed Friedel-Crafts Alkylation Reaction
Guo, Chang-Qiu,Liu, Ren-Rong,Lu, Chuan-Jun,Wang, Xiao-Mei,Xu, Qi,Zhang, De-Bing,Zhang, Peng
supporting information, p. 15005 - 15010 (2021/09/30)
Access to α,α-dihaloacetophenones through anodic C[dbnd]C bond cleavage in enaminones
Bu, Jiping,Huang, Zijun,Li, Shaoke,Ma, Xiantao,Wu, Kairui,Yang, Jiusi,Yu, Renjie,Zhang, Zhenlei
, (2021/12/20)
We have developed a method to synthesize α,α-dihaloketones under electrochemical conditions. The electrosynthesis reaction can be conveniently carried out in an undivided electrolytic cell at room temperature. In addition, various functional groups are compatible with this green protocol which can be applied simultaneously to the gram scale without significantly lower yield.
1451-82-7 Upstream products
-
5337-93-9
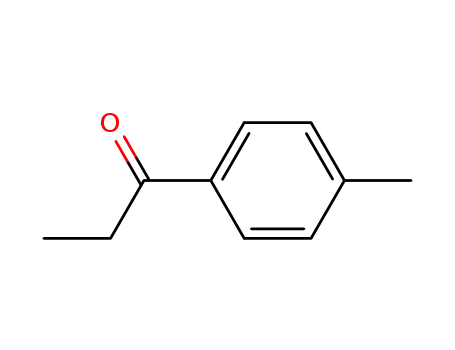
4'-methylpropiophenone
-
563-76-8
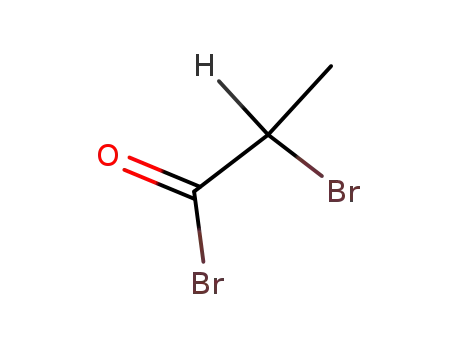
α-bromopropionyl bromide
-
108-88-3
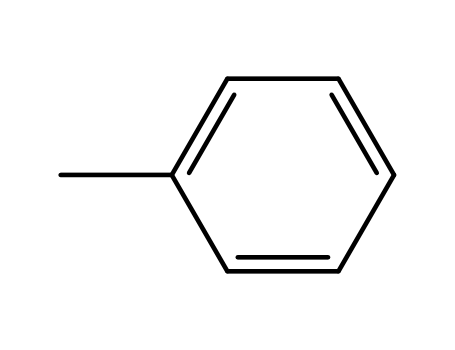
toluene
-
7726-95-6
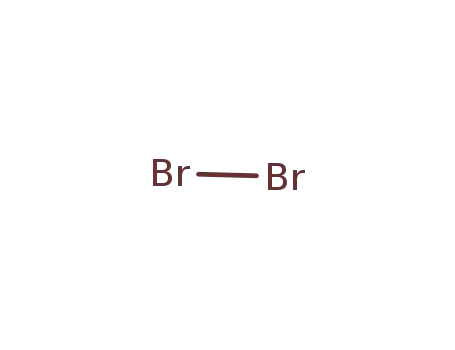
bromine
1451-82-7 Downstream products
-
15482-26-5
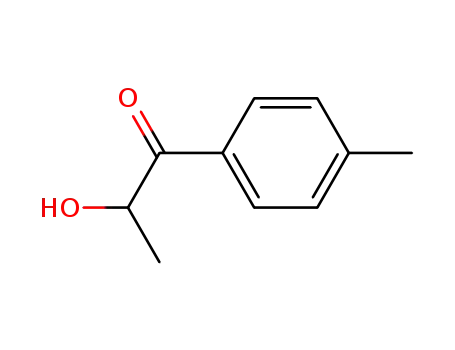
2-hydroxy-1-(4-methylphenyl)propan-1-one
-
236117-38-7
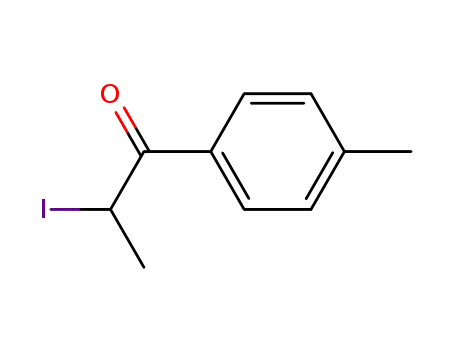
2-iodo-1-(p-tolyl)propan-1-one
-
64403-76-5
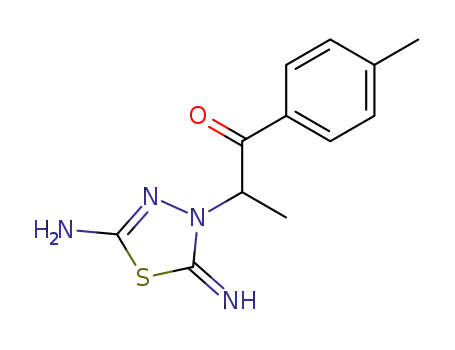
2-(5-amino-2-imino-[1,3,4]thiadiazol-3-yl)-1-p-tolyl-propan-1-one
-
1451-87-2
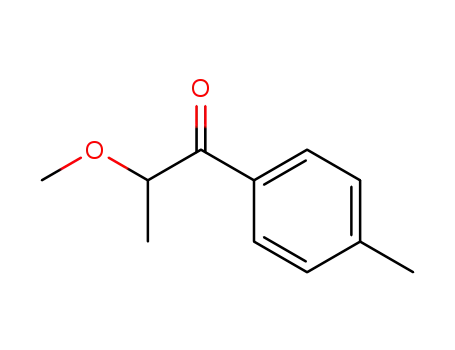
2-methoxy-1-p-tolylpropan-1-one
Relevant Products
-
Phenol,4-bromo-2-chloro- 3964-56-5
CAS:3964-56-5
-
Coenzyme Q10
CAS:303-98-0
-
Lidocaine
CAS:137-58-6