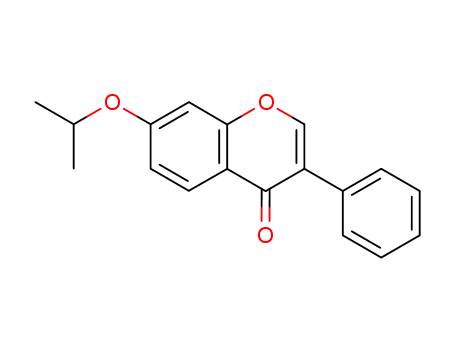
Product Details
Appearance:white crystalline powder
Reliable quality Ipriflavone 35212-22-7 global trade, in bulk supply
- Molecular Formula:C18H16O3
- Molecular Weight:280.323
- Appearance/Colour:white crystalline powder
- Vapor Pressure:8.47E-08mmHg at 25°C
- Melting Point:116-120 °C(lit.)
- Refractive Index:1.592
- Boiling Point:435.9 °C at 760 mmHg
- Flash Point:209.3 °C
- PSA:39.44000
- Density:1.184 g/cm3
- LogP:4.24720
35212-22-7 Usage
Ipriflavone (IP), one of the anti-catabolic agents, is a synthesized derivative of isoflavone that is found abundantly in plants. IP has been reported to be effective in the prevention and treatment of OP, which works by increasing bone formation by both inhibiting parathyroid hormone (PTH)-stimulated bone resorption and the maturation of osteoclast cells, and enhancing alkaline phosphatase (ALP) activity in osteoblast cells. IP has also been reported to increase BMD and reduce bone turnover rates.
Definition
ChEBI: A member of the class of isoflavones that is isoflavone in which the hydrogen at position 7 is replaced by an isopropoxy group. A synthetic isoflavone, it was formerly used for the treatment of osteoporosis, although a randomised controlled study failed to show any benefit. It is still used to prevent osteoporosis in post-menopausal women.
InChI:InChI=1/C18H16O3/c1-12(2)21-14-8-9-15-17(10-14)20-11-16(18(15)19)13-6-4-3-5-7-13/h3-12H,1-2H3
35212-22-7 Relevant articles
Ipriflavone as a non-steroidal glucocorticoid receptor antagonist ameliorates diabetic cognitive impairment in mice
Ruifang Nie, Jian Lu, Rui Xu, Juanzhen Yang, Xingyi Shen, Xingnan Ouyang, Danyang Zhu, Yujie Huang, Tong Zhao, Xuejian Zhao, Yin Lu, Minyi Qian, Jiaying Wang, Xu Shen
, Aging Cell Volume21, Issue3 March 2022
Here, we determined that ipriflavone (IP) a clinical anti-osteoporosis drug functioned as a non-steroidal GR antagonist and efficiently ameliorated learning and memory dysfunction in both type 1 and 2 diabetic mice.
In vivo Ipriflavone Mutagenicity and Cytotoxicity after Repeated Treatment Doses
Jean Carlos Vencioneck Dutra , Paula Roberta Costalonga Pereira , Juliana Macedo Delarmelina , Luciano Belcavello and Maria do Carmo Pimentel Batitucci
Journal of Pharmacy and Pharmacology 10 (2022) 313-315
For the conditions tested, the treatment clinic dose (8.57 mg.kg-1 ) was the only one that did not induce mutagenic or cytotoxic damage. Our findings reinforce the safe use of ipriflavone and corroborate those found in the literature.
The efficacy and safety of ipriflavone in postmenopausal women with osteopenia or osteoporosis: A systematic review and meta-analysis
Qinsheng Hu a 1, Cheng Long a 1, Diwei Wu a, Xuanhe You a, Liyu Ran a, Jiazhuang Xu b, Eric O Klineberg c, Shishu Huang a, Jiali Chen a, Ning Ning a
Pharmacological Research Volume 159, September 2020, 104860
Ipriflavone (IP) is one of the over-the-counter drugs and found in foods, which is available for prevention of osteoporosis (OP) since 1989 in over 22 countries. Although some clinical trials have suggested that IP is appropriate for treatment of OP, there continues to be controversy regarding the efficacy and safety due to some contradictory reports. With the wide usage of IP for osteoporotic women, there is a critical need for evaluation of the evidence for IP in clinical practice.
35212-22-7 Upstream products
-
75-30-9
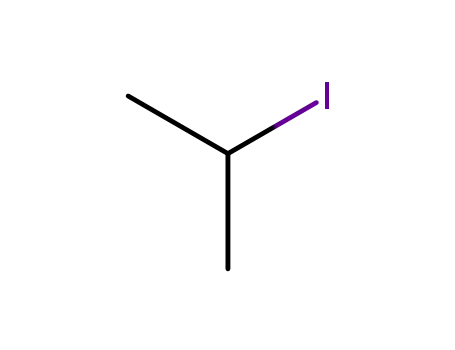
2-iodo-propane
-
13057-72-2
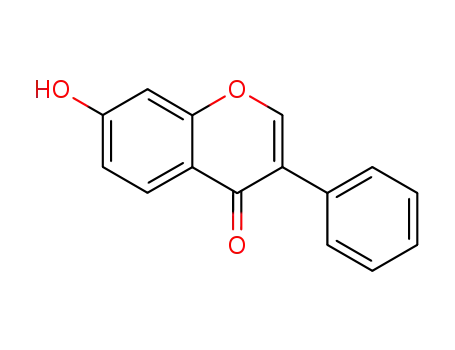
7-hydroxyisoflavone
-
75-26-3
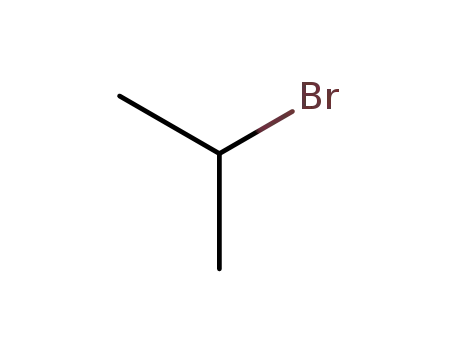
isopropyl bromide
-
3669-41-8
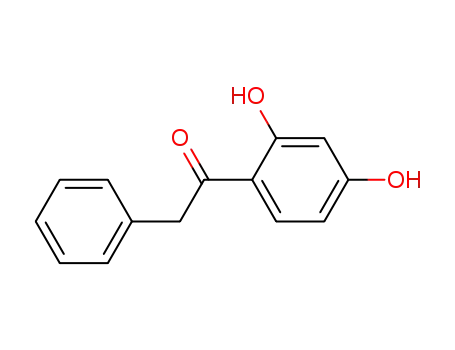
1-(2,4-dihydroxyphenyl)-2-phenylethanone
35212-22-7 Downstream products
-
163618-93-7
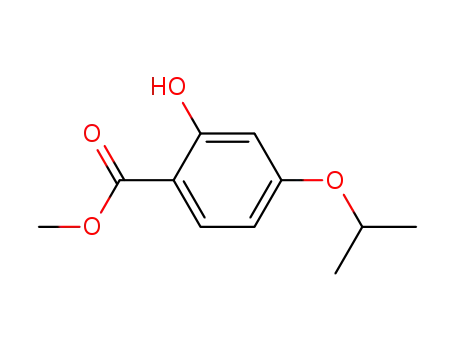
methyl 2-hydroxy-4-isopropyl benzoate
-
1179998-08-3
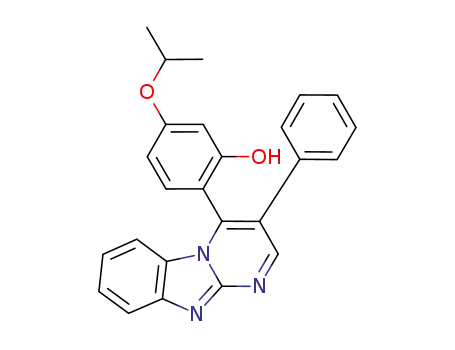
2-(2-hydroxy-4-isopropoxyphenyl)-3-phenyl-pyrimido[1,2-a]benzimidazole
-
1233690-14-6
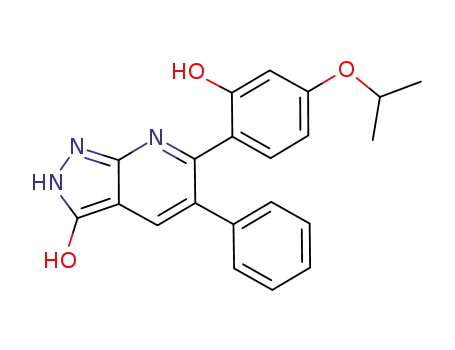
3-hydroxy-5-phenyl-6-(2-hydroxy-4-isopropoxyphenyl)-1H-pyrazolo[3,4-b]pyridine
-
1232561-77-1
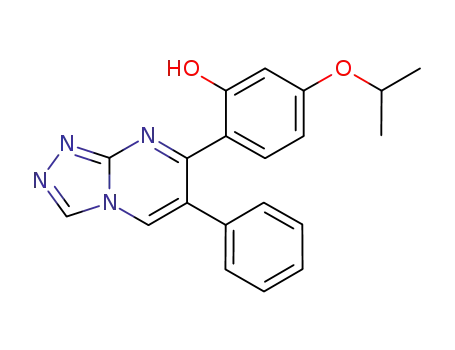
6-phenyl-7-(2-hydroxy-4-isopropoxyphenyl)-[1,2,4]triazolo[4,3-a]pyrimidine
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
Methenolone Acetate
CAS:434-05-9
-
Coenzyme Q10
CAS:303-98-0