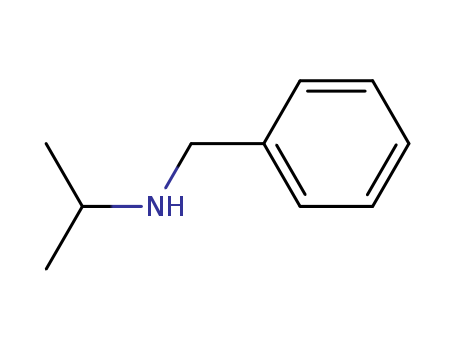
102-97-6
- Product Name:N-Benzylisopropylamine
- Molecular Formula:C10H15N
- Purity:99%
- Molecular Weight:
Product Details
Appearance:Clear, very slightly yellow to yellow liquid
Shanghai Upbio Tech Co.,Ltd (Former Onchem (China)Co.,Ltd) is a comprehensive manufacturer and an international distribution of chemicals throughout the world. The service purpose of our company is the principle based on good faith and costumers' satisfaction. from the natural, health services for the idea. Services to global customers. Strive to build the world's leading pharmaceutical,health food industry raw material suppliers.
N-Benzylisopropylamine 102-97-6 supplier and good producer
- Molecular Formula:C10H15N
- Molecular Weight:149.236
- Appearance/Colour:Clear, very slightly yellow to yellow liquid
- Vapor Pressure:0.332mmHg at 25°C
- Melting Point:143°C (estimate)
- Refractive Index:n20/D 1.502(lit.)
- Boiling Point:199.999 °C at 760 mmHg
- PKA:9.77±0.19(Predicted)
- Flash Point:87.778 °C
- PSA:12.03000
- Density:0.907 g/cm3
- LogP:2.57550
N-Benzylisopropylamine 102-97-6 Usage
N-Benzylisopropylamine, an isomer of methamphetamine, has been used to adulterate methamphetamine, and distributed as fake “Ice”. N-Isopropylbenzylamine was used as ligand in the preparation and characterization of bis(cyclopentadienyl)magnesium. It was also used in the synthesis of N-benzylideneisopropylamine-N-oxide.
InChI:InChI=1/C10H15N/c1-9(2)11-8-10-6-4-3-5-7-10/h3-7,9,11H,8H2,1-2H3/p+1
102-97-6 Relevant articles
N-isopropylbenzylamine, a methamphetamine mimics, produces toxicity via increasing nitric oxide in vitro
Peng Xu , Haijie Li , Qiyang Qiu , Xiao Xiao , Yi Qiu , Xiangyu Li , Youmei Wang , Wenhua Zhou , Haowei Shen , Wei Cui
Toxicology Volume 480, October 2022, 153337
We have found, for the first time, that N-isopropylbenzylamine produced toxicity in cell lines that model neurons. And the toxicity of this compound might be at least partially related to the activation of nNOS, and the production of intracellular NO.
Stereoselective pinacol coupling of planar chiral (benzaldehyde)Cr(Co)3, (benzaldimine)Cr(CO)3, ferrocenecarboxaldehyde and (dienal)Fe(CO)3 complexes with samarium diiodide
Taniguchi, Nobukazu,Uemura, Motokazu
, p. 12775 - 12788 (1998)
An intermolecular pinacol coupling of the Planar chiral tricarbonylchromium complexes of o-substituted benzaldehydes or benzaldimines with samarium(II) diiodide in THF produces exclusively threo 1.2-diols or 1,2-diamines in an optically pure form, while the corresponding racemic o- substituted benzaldehyde or benzaldimine chromium complexes give a mixture of threo and erythro pinacol coupling products in a various ratio depending upon the nature of o-substituent. Similarly, planar chiral 2-substituted ferrocenecarboxaldehydes and (dienal)Fe(CO)3 produce the corresponding 1.2- diols with high stereoselectivity. The generated transition metal-complexed ketyl radical intermediates are configurationally stable with restriction to a rotation about C(α)-C(ipso) bond.
Design of a bifunctional Ir-Zr based metal-organic framework heterogeneous catalyst for the N-alkylation of amines with alcohols
Rasero-Almansa,Corma,Iglesias,Sanchez
, p. 1794 - 1800 (2014)
The direct N-alkylation of amines with alcohols was performed with an Ir-Zr-based metal-organic framework multifunctional heterogeneous catalyst. This system is efficient and environmentally benign for the synthesis of various organic amines in air in the absence of a base. The catalyst was recovered and reused without significant loss of activity, and only water was produced as a byproduct. Better be direct than elusive: The direct N-alkylation of amines with alcohols is performed with an Ir-Zr-based metal-organic framework multifunctional heterogeneous catalyst. This system is efficient and environmentally benign for the synthesis of various organic amines in air in the absence of a base. The catalyst is recovered and reused without significant loss of activity, and only water is produced as a byproduct.
First example of direct reductive amination of aldehydes with primary and secondary amines catalyzed by water-soluble transition metal catalysts
Robichaud, André,Nait Ajjou, Abdelaziz
, p. 3633 - 3636 (2006)
An unprecedented efficient and highly selective direct reductive amination of aldehydes with primary and secondary amines in water using gaseous hydrogen and water-soluble catalysts is developed. The catalytic system formed in situ from Pd(PhCN)2Cl2 and 2,2′-biquinoline-4,4′-dicarboxylic acid dipotassium salt (BQC), allows full conversion of aldehydes and the formation of desired alkylated amines with excellent yields and selectivities. The catalytic system is stable and can be recycled and reused three times without loss of activity.
Expanding Water/Base Tolerant Frustrated Lewis Pair Chemistry to Alkylamines Enables Broad Scope Reductive Aminations
Fasano, Valerio,Ingleson, Michael J.
, p. 2217 - 2224 (2017)
Lower Lewis acidity boranes demonstrate greater tolerance to combinations of water/strong Br?nsted bases than B(C6F5)3, this enables Si?H bond activation by a frustrated Lewis pair (FLP) mechanism to proceed in the presence of H2O/alkylamines. Specifically, BPh3has improved water tolerance in the presence of alkylamines as the Br?nsted acidic adduct H2O–BPh3does not undergo irreversible deprotonation with aliphatic amines in contrast to H2O–B(C6F5)3. Therefore BPh3is a catalyst for the reductive amination of aldehydes and ketones with alkylamines using silanes as reductants. A range of amines inaccessible using B(C6F5)3as catalyst, were accessible by reductive amination catalysed by BPh3via an operationally simple methodology requiring no purification of BPh3or reagents/solvent. BPh3has a complementary reductive amination scope to B(C6F5)3with the former not an effective catalyst for the reductive amination of arylamines, while the latter is not an effective catalyst for the reductive amination of alkylamines. This disparity is due to the different pKavalues of the water–borane adducts and the greater susceptibility of BPh3species towards protodeboronation. An understanding of the deactivation processes occurring using B(C6F5)3and BPh3as reductive amination catalysts led to the identification of a third triarylborane, B(3,5-Cl2C6H3)3, that has a broader substrate scope being able to catalyse the reductive amination of both aryl and alkyl amines with carbonyls.
Reductive amination of ketones/aldehydes with amines using BH3N(C2H5)3as a reductant
Zou, Qizhuang,Liu, Fei,Zhao, Tianxiang,Hu, Xingbang
supporting information, p. 8588 - 8591 (2021/09/04)
Herein, we report the first example of efficient reductive amination of ketones/aldehydes with amines using BH3N(C2H5)3 as a catalyst and a reductant under mild conditions, affording various tertiary and secondary amines in excellent yields. A mechanistic study indicates that BH3N(C2H5)3 plays a dual function role of promoting imine and iminium formation and serving as a reductant in reductive amination. This journal is
BF3·Et2O as a metal-free catalyst for direct reductive amination of aldehydes with amines using formic acid as a reductant
Fan, Qing-Hua,Liu, Xintong,Luo, Zhenli,Pan, Yixiao,Xu, Lijin,Yang, Ji,Yao, Zhen,Zhang, Xin
supporting information, p. 5205 - 5211 (2021/07/29)
A versatile metal- and base-free direct reductive amination of aldehydes with amines using formic acid as a reductant under the catalysis of inexpensive BF3·Et2O has been developed. A wide range of primary and secondary amines and diversely substituted aldehydes are compatible with this transformation, allowing facile access to various secondary and tertiary amines in high yields with wide functional group tolerance. Moreover, the method is convenient for the late-stage functionalization of bioactive compounds and preparation of commercialized drug molecules and biologically relevant N-heterocycles. The procedure has the advantages of simple operation and workup and easy scale-up, and does not require dry conditions, an inert atmosphere or a water scavenger. Mechanistic studies reveal the involvement of imine activation by BF3and hydride transfer from formic acid.
102-97-6 Upstream products
-
100-39-0
benzyl bromide
-
75-31-0
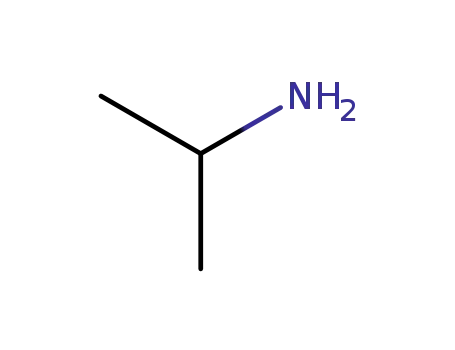
isopropylamine
-
72846-70-9
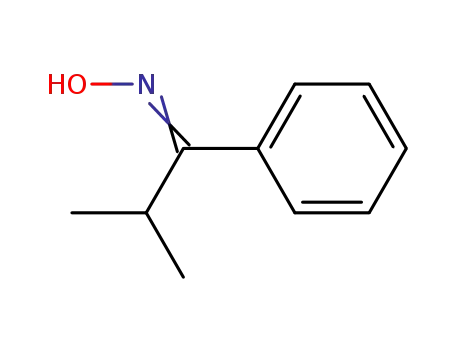
isopropyl phenyl ketoxime
-
100-44-7
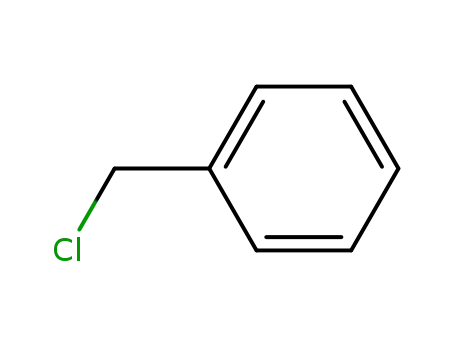
benzyl chloride
102-97-6 Downstream products
-
87040-39-9
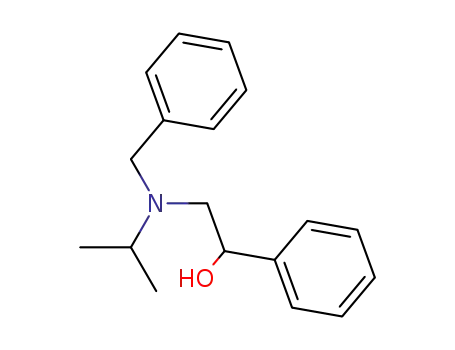
(+/-)-benzylisopropyl(α-hydroxyphenethyl)amine
-
27171-30-8
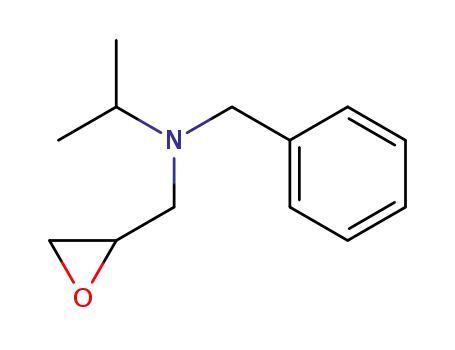
N-benzyl-N-isopropyl-2,3-epoxypropylamine
-
20278-21-1
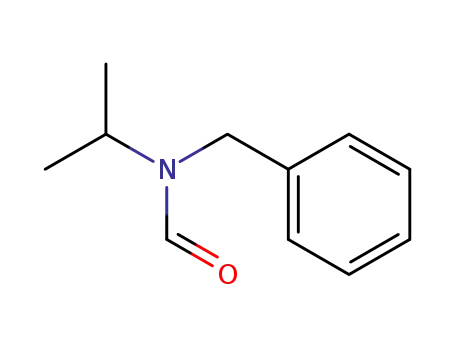
N-Benzyl-N-isopropylformamid (anti)
-
60882-81-7
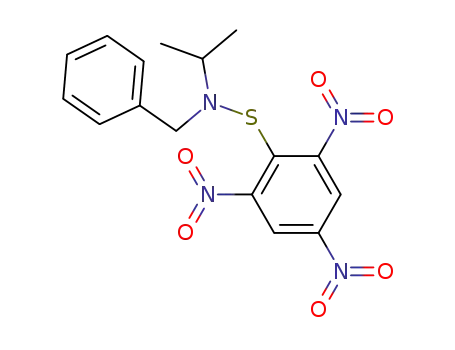
N-2-Propyl-N-benzyl-2,4,6-trinitrobenzolsulfenamid
Relevant Products
-
Cyclopropane, isocyanato- 4747-72-2
CAS:4747-72-2
-
2-Thiouracil
CAS:141-90-2
-
Methyltetrahydrophthalic anhydride
CAS:11070-44-3