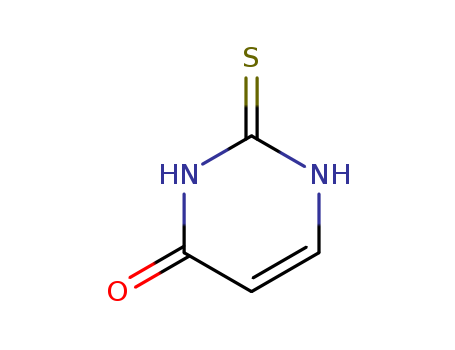
Product Details
Appearance:white to cream fine crystalline powder
2-Thiouracil 141-90-2 manufacturer, in stock, low price
- Molecular Formula:C4H4N2OS
- Molecular Weight:128.155
- Appearance/Colour:white to cream fine crystalline powder
- Vapor Pressure:5.45E-05mmHg at 25°C
- Melting Point:>300 °C(lit.)
- Refractive Index:1.677
- Boiling Point:337.2 °C at 760 mmHg
- PKA:pKa 7.46 (Uncertain)
- Flash Point:157.7 °C
- PSA:80.74000
- Density:1.46 g/cm3
- LogP:0.43250
2-Thiouracil 141-90-2 Usage
2-Thiouracil is a chemical derivative of uracil in which the oxygen atom in the 2-position of the ring is substituted by sulfur. It is white to cream fine crystalline powder. It acts as an anticancer, antithyroid, and antiviral agent.
Definition
ChEBI: A nucleobase analogue that is uracil in which the oxo group at C-2 is replaced by a thioxo group.
Purification Methods
Crystallise 2-thiouracil from water or EtOH.
InChI:InChI=1/C4H4N2OS/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8)
Shanghai Upbio Tech Co.,Ltd (Former Onchem (China)Co.,Ltd) is a comprehensive manufacturer and an international distribution of chemicals throughout the world, The predecessor of Shanghai Upbio Tech Co.,Ltd was Onchem (China) Co.,Ltd in 2010,Specialized in APIs, chemical intermediates, herbal extract and pharmaceutical raw materials.
We have our own GMP factory with R&D centure.and cleaning workshop , and some main products produced by our own lab and R&D centure.besides we have established very good cooperation and business relations with hundreds of Chinese qualified manufacturers by long-term business intercourse.
141-90-2 Relevant articles
A simple strategy to enhance the luminescence of metal nanoclusters and its application for turn-on detection of 2-thiouracil and hyaluronidase
Miao Liang , Zhongli Lei , Yiling Li, Yan Xiao
Talanta Volume 236, 1 January 2022, 122876
Since 2-thiouracil (2-TU), a common anticancer, antithyroid, and antiviral agent, featured a similar molecular structure of ATT, this luminescence enhancement strategy can be designed to sensitive and selective turn-on detect 2-TU.
Kinetics and adsorption isotherm model of 2-thiouracil adsorbed onto the surface of reduced graphene oxide-copper oxide nanocomposite material
Pramanand Kumar, Subrata Das
Journal of Molecular Structure Volume 1268, 15 November 2022, 133723
Thiouracil is a nucleobase analogue that is uracil in which the oxo group at C-2 is replaced by a thioxo group. It has a role as an antithyroid drug and a metabolite. We report the synthesis of rGO-CuO using the chemical modification method, characterization, and the adsorption isotherm behavior of the nanocomposite material for the removal of 6-amino-2-thiouracil (6A2TU) and 6-amino-5-nitroso-2-thiouracil (6A5NTU) in an aqueous medium.
141-90-2 Upstream products
-
6965-19-1
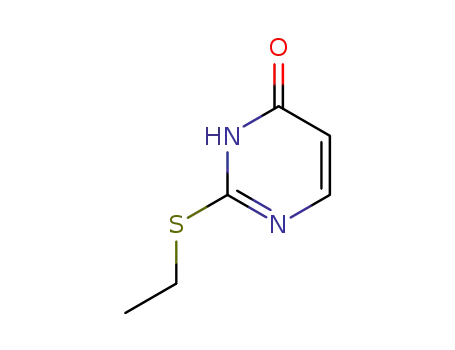
2-ethylthio-3H-pyrimidin-4-one
-
98484-57-2
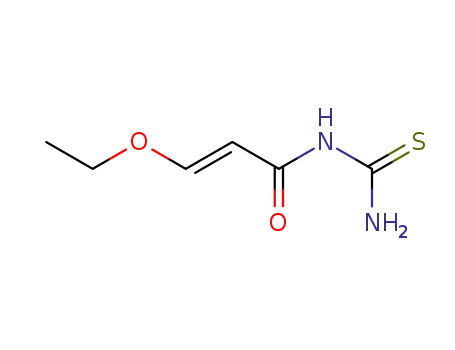
(3t-ethoxy-acryloyl)-thiourea
-
10601-80-6
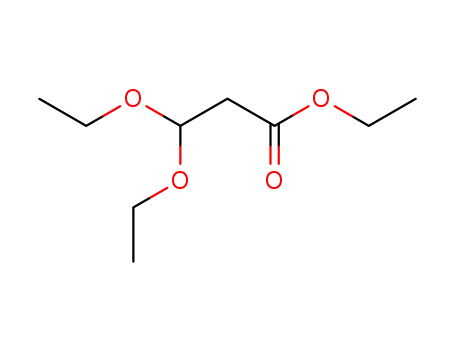
ethyl 3,3-diethoxypropanoate
-
17356-08-0
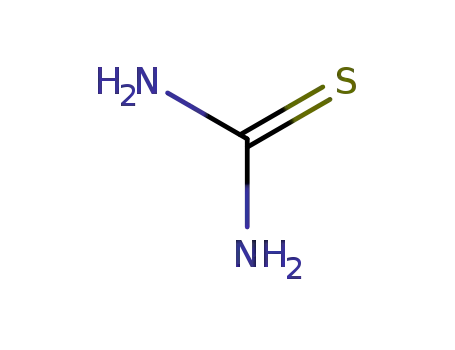
thiourea
141-90-2 Downstream products
-
21052-18-6
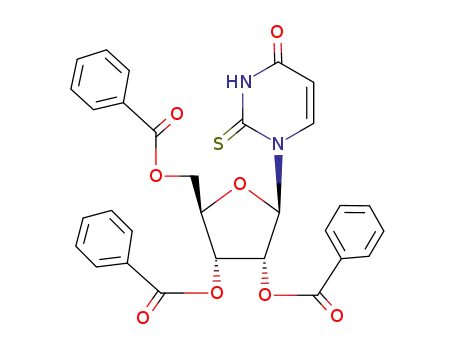
2-thio-1-(2',3',5'-tri-O-benzoyl-β-D-ribofuranosyl)-(3H)pyrimidine-2,4-dione
-
5751-20-2
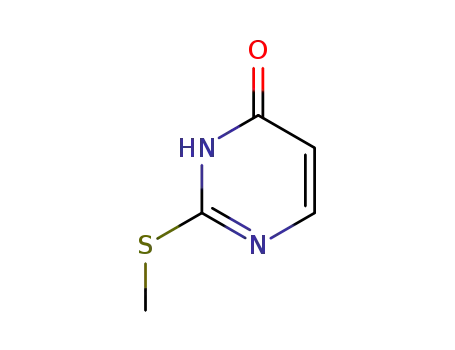
2-Methylthiouracil
-
6327-98-6
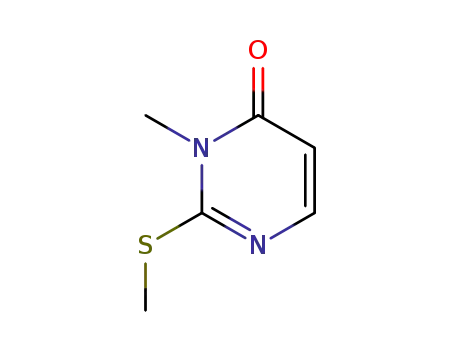
2-methylthio-3-methylpyrimidin-4(3H)-one
-
6330-98-9
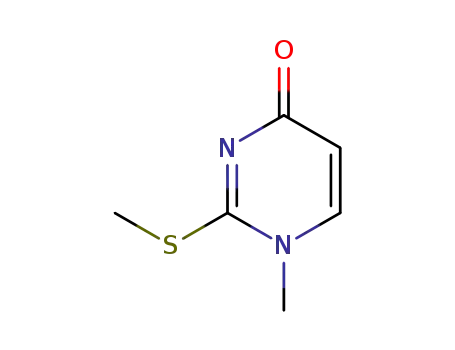
1-methyl-2-methysulfanylpyrimidin-4(1H)-one
Relevant Products
-
Sparteine sulfate anhydrous
CAS:299-39-8
-
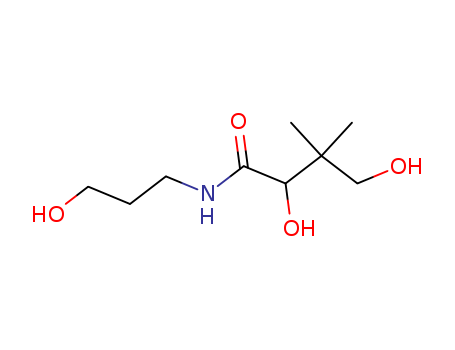
DL-Panthenol,Panthenol
CAS:16485-10-2
-
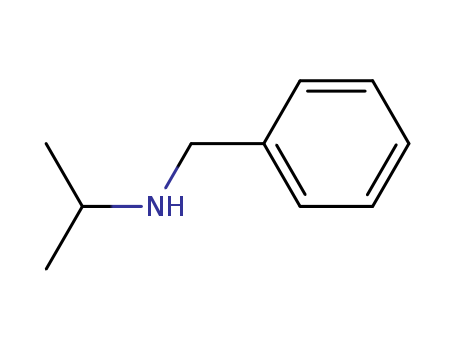
N-Benzylisopropylamine
CAS:102-97-6