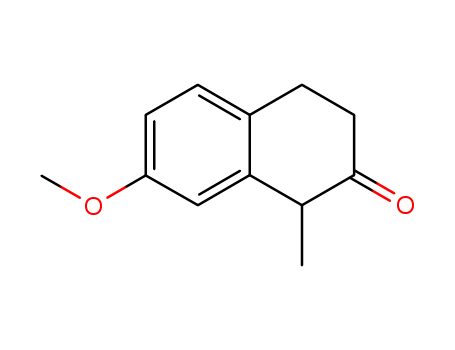
1204-23-5
- Product Name:2(1H)-Naphthalenone, 3,4-dihydro-7-methoxy-1-methyl-
- Molecular Formula:C12H14O2
- Purity:99%
- Molecular Weight:
Product Details
1204-23-5 for sale with reasonable price, 7-Methoxy-1-methyl-2-tetralone trader
- Molecular Formula:C12H14O2
- Molecular Weight:190.242
- Boiling Point:125-126 °C(Press: 0.8 Torr)
- PSA:26.30000
- Density:1.076±0.06 g/cm3(Predicted)
- LogP:2.31400
1204-23-5 Usage
7-Methoxy-1-methyl-2-tetralone is a chiral, alkylating agent that is used in the synthesis of cinchonidine. It has been synthesized by the reaction of methylbenzene with diethyl ether and hydroxylamine, followed by transfer to chloroform. The product can be purified by recrystallization from benzene and leaves. This compound can be synthesized stereoselectively by using an asymmetric synthesis with a catalyst such as benzocyclodecene. 7-Methoxy-1-methyl-2-tetralone is a reagent that is used in the synthesis of dezocine (D299800), which is an opioid analgesic that is related to Pentazocine (P274300).
1204-23-5 Relevant articles
PHARMACEUTICALLY ACCEPTABLE SALTS OF BENZODICYCLOALKANE DERIVATIVE, POLYMORPHIC SUBSTANCE THEREOF, AND APPLICATION THEREOF
-
Paragraph 0211; 0215; 0228, (2021/04/02)
The present invention provides a pharmaceutically acceptable salt of benzodicycloalkane derivative and a polymorph thereof, and an application thereof. Specifically, the present invention provides a polymorph of benzobicyclic alkane derivative or a pharma
BENZODICYCLOALKANE DERIVATIVE, PREPARATION METHOD AND USE THEREOF
-
Paragraph 0155; 0158, (2019/06/19)
It is provided herein a benzobicycloalkane derivative, and a preparation method and use thereof. In particular, it is provided herein a compound of Formula (I), or a pharmaceutically acceptable salt, stereoisomer or solvate thereof, a preparation method, and a use thereof in preparation of drugs for treating pain.
A key intermediate for the preparation method of Eptazocine
-
Paragraph 0019-0020; 0022-0023, (2017/11/16)
The present invention discloses a dezocine key intermediate preparation method, 7-methoxy-2-tetralone is used as a starting material for first benzyl-site methylation and then ortho alkylation of the carbonyl group, the dezocine key intermediate can be prepared by three strategies of stepwise synthesis, two-step and one-pot methods. The dezocine key intermediate preparation method has the advantages of simple synthetic route, less steps, high yield simple operation, great reduction of the synthesis cycle, mild reaction conditions, and improvement of the safety of the technology, the low raw material cost and easy industrialization. The preparation cost is greatly reduced by the simple synthesis method, the patient dosage cost is reduced, the national social security spending can be reduced to some extent, and some of the social and economic benefits are produced.
1204-23-5 Upstream products
-
30021-91-1
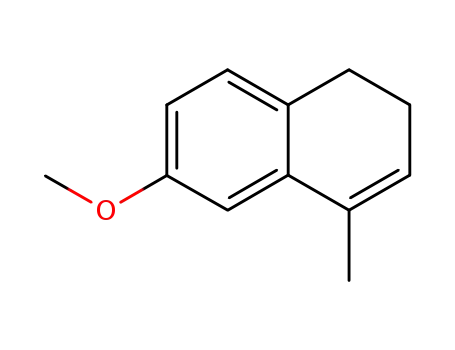
6-methoxy-4-methyl-1,2-dihydronaphthalene
-
1206-44-6
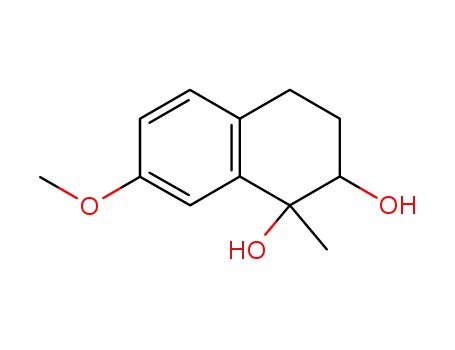
1,2-Dihydroxy-7-methoxy-1-methyl-tetralin
-
6836-19-7
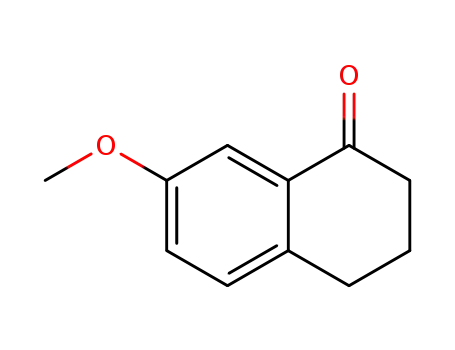
7-Methoxy-1-tetralone
-
4521-28-2
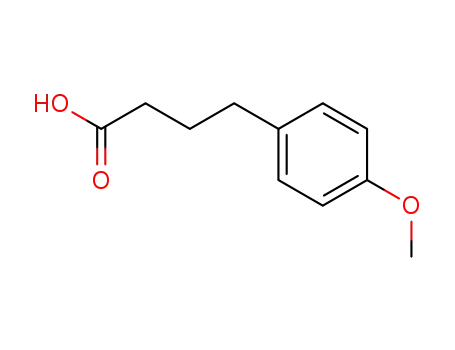
4-(4-Methoxyphenyl)butyric acid
1204-23-5 Downstream products
-
152564-71-1
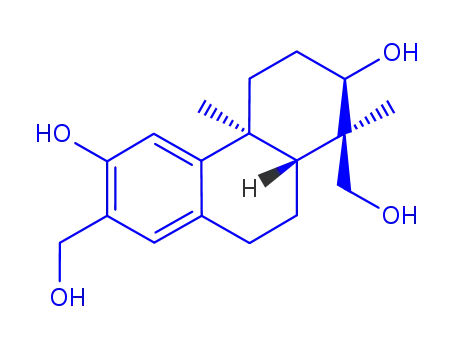
(+)-(1S,2R,4aS,10aR)-1,7-bis(hydroxymethyl)-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-2,6-phenanthrenediol
-
152564-71-1
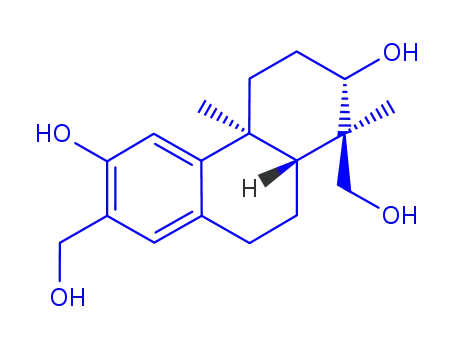
(+)-(1S,2S,4aS,10aR)-1,7-bis(hydroxymethyl)-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-2,6-phenanthrenediol
-
152564-71-1
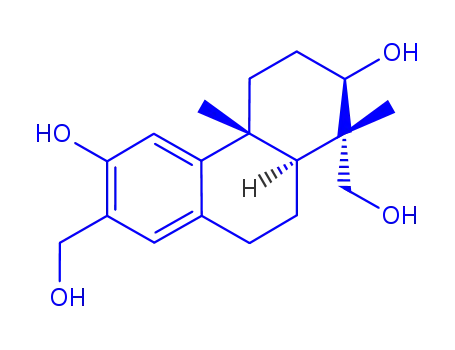
(-)-(1R,2R,4aR,10aS)-1,7-bis(hydroxymethyl)-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-2,6-phenanthrenediol
-
152564-71-1
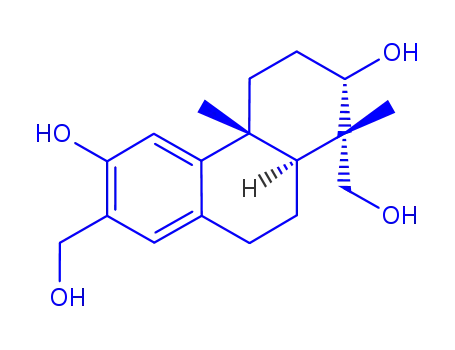
(-)-(1R,2S,4aR,10aS)-1,7-bis(hydroxymethyl)-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-2,6-phenanthrenediol
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
Prilocaine
CAS:721-50-6
-
Echinacoside
CAS:82854-37-3