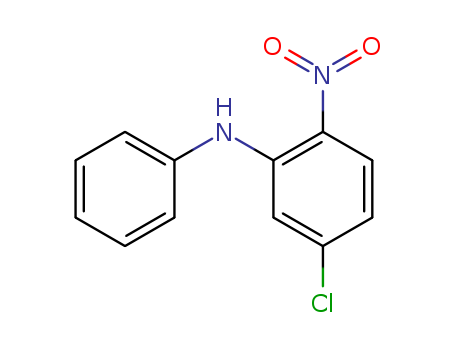
25781-92-4
- Product Name:5-Chloro-2-nitrodiphenylamine
- Molecular Formula:C12H9ClN2O2
- Purity:99%
- Molecular Weight:
Product Details
Appearance:orange-red fine crystalline powder
5-Chloro-2-nitrodiphenylamine good producer, 25781-92-4 supplier with low price
- Molecular Formula:C12H9ClN2O2
- Molecular Weight:248.669
- Appearance/Colour:orange-red fine crystalline powder
- Vapor Pressure:1.11E-05mmHg at 25°C
- Melting Point:110-114 °C
- Refractive Index:1.671
- Boiling Point:370.414 °C at 760 mmHg
- PKA:-4.38±0.50(Predicted)
- Flash Point:177.82 °C
- PSA:57.85000
- Density:1.387 g/cm3
- LogP:4.58800
5-Chloro-2-nitrodiphenylamine 25781-92-4 Usage
5-Chloro-2-nitrodiphenylamine is a synthetic dyestuff that belongs to the class of acridones. It can be used as an anti-epileptic drug. It was also used as the starting material for the preparation of substituted Phenylbenzimidazoles.
InChI:InChI=1/C12H9ClN2O2/c13-9-6-7-12(15(16)17)11(8-9)14-10-4-2-1-3-5-10/h1-8,14H
25781-92-4 Relevant articles
Antitubercular Substances: XXI. Synthesis of 2-Nitrodiphenylamines
J. G. Belton and Mary McInerney
Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science Vol. 69 (1970), pp. 21-29 (9 pages)
SYNTHETIC methods for 2-nitrodiphenylamines and N-cyclohexyl-2-nitroanilines are described.
A 1,5-benzodiazepine derivatives zhuo Tong method for the preparation of (by machine translation)
-
Paragraph 0025; 0026; 0027; 0028; 0041, (2016/11/02)
The method step of this invention is simple, cheap material, easy to operate and achieve commercial, yield and purity are greatly improved. (by machine translation)
Oxidative nucleophilic aromatic amination of nitrobenzenes
Khutorianskyi,Sonawane,Po?ta,Klepetá?ová,Beier
supporting information, p. 7237 - 7240 (2016/06/09)
Nitrobenzenes substituted with electron-acceptor groups such as halogen, nitro, trifluoromethyl, pentafluorosulfanyl, or cyano underwent oxidative nucleophilic substitution with lithium salts of arylamines to afford N-aryl-2-nitroanilines.
The ferric chloride oxidation of 5-substituted o-semidines and the polarographic properties of the products
AP Kottenhahn, ET Seo, HW Stone
The Journal of Organic Chemistry, 1963
A solution of 102 g. (0.5 mole) of l-ehloro-3,4-dinitrobenzene and 140 ml. … (0.117 mole) of 5-chloro-2-nitrodiphenylamine in a pressure bottle was …
25781-92-4 Process route
-
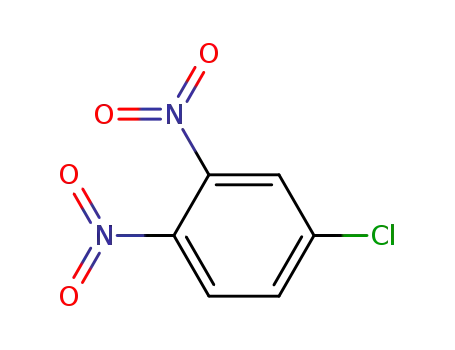
- 610-40-2
3,4-dinitro-chlorobenzene

-
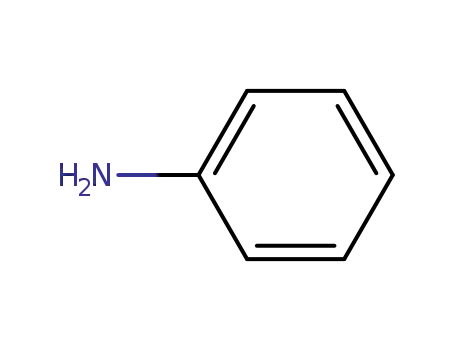
- 62-53-3
aniline

-
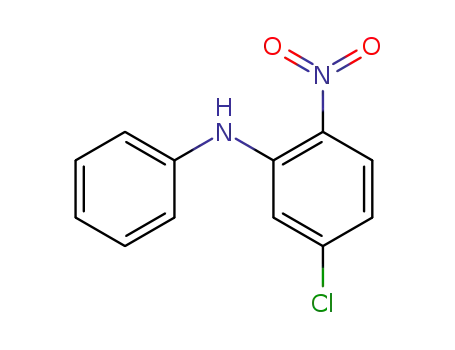
- 25781-92-4
5-chloro-2-nitro-N-phenylaniline

-
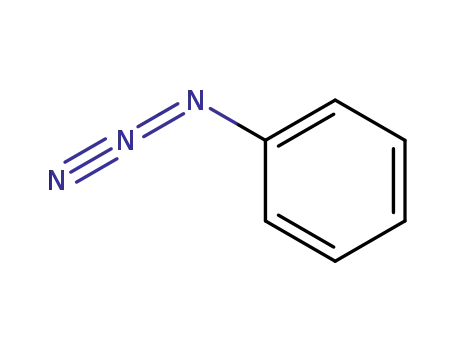
- 622-37-7
Phenyl azide
| Conditions | Yield |
|---|---|
|
|
-

- 610-40-2
3,4-dinitro-chlorobenzene

-

- 62-53-3
aniline

-

- 25781-92-4
5-chloro-2-nitro-N-phenylaniline

-
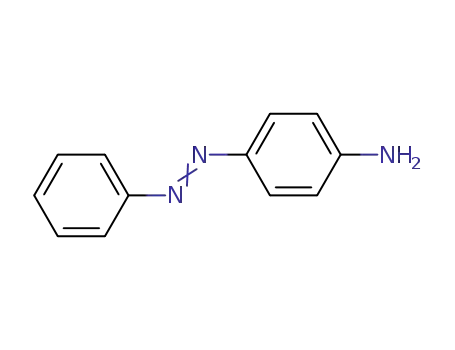
- 60-09-3
aniline yellow
| Conditions | Yield |
|---|---|
|
anschl. mit HCl;
|
|
|
|
25781-92-4 Upstream products
-
127-09-3
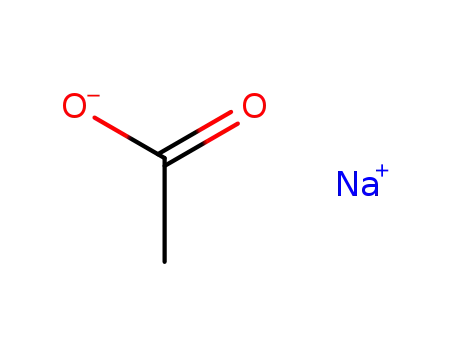
sodium acetate
-
610-40-2

3,4-dinitro-chlorobenzene
-
62-53-3

aniline
-
611-06-3
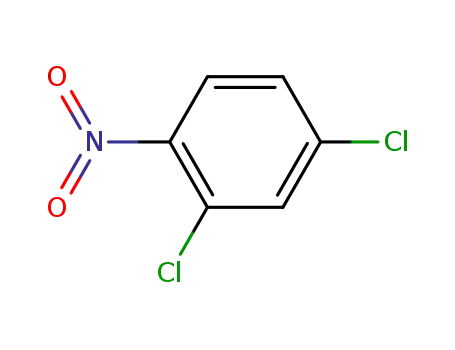
2,4-dichloronitrobenzene
25781-92-4 Downstream products
-
68406-47-3
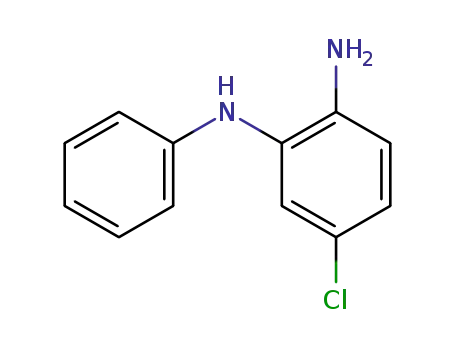
4-chloro-N2-phenylbenzene-1,2-diamine
-
861342-91-8
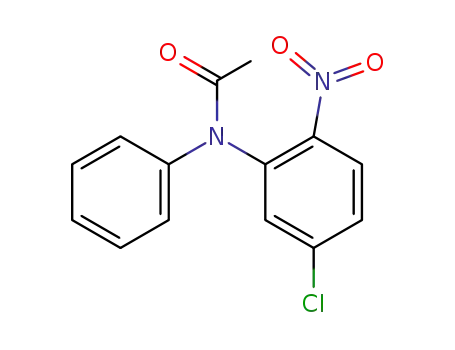
N-(5-chloro-2-nitro-phenyl)-N-phenyl-acetamide
-
61154-60-7
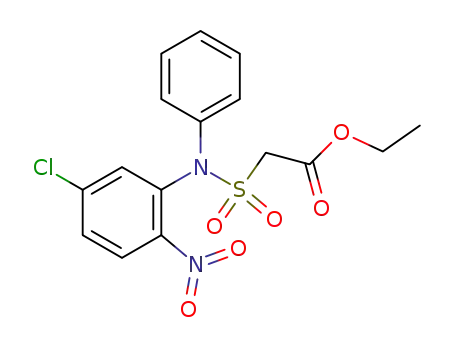
[(5-Chloro-2-nitro-phenyl)-phenyl-sulfamoyl]-acetic acid ethyl ester
-
54986-47-9
6-chloro-1,3-dihydro-1-phenyl-2H-benzimidazol-2-one
Relevant Products
-
2-Bromo-9,9-dimethylfluorene
CAS:28320-31-2
-
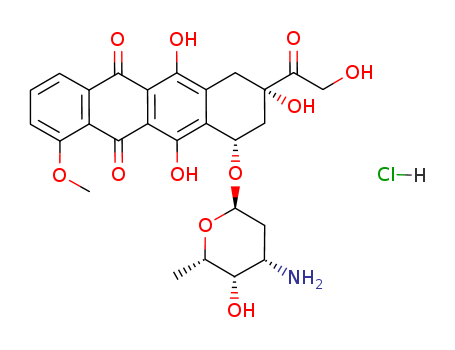
Doxorubicin hydrochloride
CAS:25316-40-9