28320-31-2
- Product Name:2-Bromo-9,9-dimethylfluorene
- Molecular Formula:C15H13Br
- Purity:99%
- Molecular Weight:
Product Details
Appearance:off kind of white crystallization
Factory sells 9-dimethylfluorene CAS NO.28320-31-2 in bulk supply with best price
- Molecular Formula:C15H13Br
- Molecular Weight:273.172
- Appearance/Colour:off kind of white crystallization
- Vapor Pressure:0mmHg at 25°C
- Melting Point:68 °C
- Refractive Index:1.615
- Boiling Point:352.9 °C at 760 mmHg
- Flash Point:165.097 °C
- PSA:0.00000
- Density:1.346 g/cm3
- LogP:4.75540
9-dimethylfluorene 28320-31-2 Usage
9-dimethylfluorene is an organic compound with specific chemical properties. It appears as a pale yellow to white crystalline powder at room temperature and has relatively stable physical properties. Regarding its physical properties, the melting point of 9-dimethylfluorene is about 96°C to 97°C, and the boiling point is in the range of 300.8±12.0°C (at 760 mmHg). Its density is about 1.0±0.1 g/cm3, and its flash point is about 138.7±10.3°C. In addition, its vapor pressure is almost 0 at 25°C, and its refractive index is 1.591. 9-dimethylfluorene can be used as a conducting polymer in the fabrication of a variety of devices which include photoelectronic devices, organic light emitting diodes (OLEDs) and organic solar cells (OSCs). In terms of safety, 9-dimethylfluorene has certain toxicity and irritation. It may cause harm to the human body through inhalation, skin contact or ingestion. Therefore, it is necessary to be extra careful when using and storing it, and follow the corresponding safety precautions, such as wearing appropriate gloves and goggles or masks.
InChI:InChI=1/C15H13Br/c1-15(2)13-6-4-3-5-11(13)12-8-7-10(16)9-14(12)15/h3-9H,1-2H3
The service purpose of Shanghai Upbio Tech Co.,Ltd (Former Onchem (China)Co.,Ltd) is the principle based on good faith and costumers' satisfaction. from the natural, health services for the idea. Services to global customers. Strive to build the world's leading pharmaceutical,health food industry raw material suppliers.
28320-31-2 Relevant articles
Excited-state modulation via alteration of the heterocyclic moiety in 9,9-dimethylfluorene-based Ir(iii) phosphorescent dopants for blue PhOLEDs
Bo-Sun Yun, So-Yoen Kim, Jin-Hyoung Kim, Sanghun Lee, Ho-Jin Son and Sang Ook Kang
J. Mater. Chem. C, 2022,10, 4196-4207
2-Bromo-9,9′-dimethylfluorene (10.0 g, 36.6 mmol), pyrazole (7.5 g, 0.11 mol), CuI(I) (1.4 g, 7.32 mmol), and K 2 CO 3 (15.2 g, 0.11 mol) were dissolved in DMF (200 mL). The mixture …
Interrupted carbonyl-olefin metathesis via oxygen atom transfer
Ludwig, Jacob R.,Watson, Rebecca B.,Nasrallah, Daniel J.,Gianino, Joseph B.,Zimmerman, Paul M.,Wiscons, Ren A.,Schindler, Corinna S.
, p. 1363 - 1369 (2018)
Some of the simplest and most powerful carbon-carbon bond forming strategies take advantage of readily accessible ubiquitous motifs: carbonyls and olefins. The complex polycyclic frameworks in this product class appear as common substructures in organic materials, bioactive natural products, and recently developed pharmaceuticals.
Methyl-restricted rotor rotation on the stator produces high-efficiency fluorescence emission: a new strategy to achieve aggregation-induced emission
H Yang, X Zhou, T Hui, Y Han, X Jiang, J Yan
RSC advances, 2019
Mesitylene, 2-bromo-9,9-dimethylfluorene and all other chemicals and reagents were purchased from Aladdin Industrial Corporation. THF was distilled under dry nitrogen immediately …
Synthesis of Multideuterated (Hetero) aryl Bromides by Ag (I)-Catalyzed H/D Exchange
Guang-Qi Hu, Jing-Wen Bai, En-Ci Li, Kai-Hui Liu, Fei-Fei Sheng, and Hong-Hai Zhang*
Org. Lett. 2021, 23, 5, 1554–1560
In addition, brominated polyarenes including 1-bromonaphthalene, 2-bromonaphthalene, 2-bromonathraquinone, and 2-bromo-9,9-dimethylfluorene all showed good H/D exchange …
28320-31-2 Upstream products
-
4569-45-3
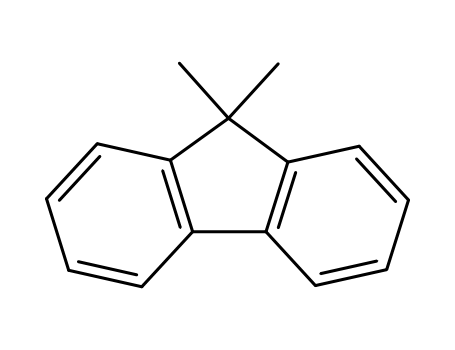
9,9-dimethyl-9H-fluorene
-
1133-80-8

2-bromo-9H-fluorene
-
74-88-4
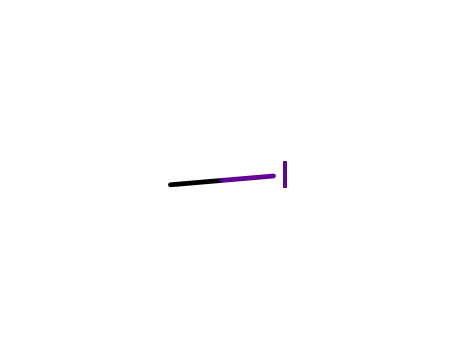
methyl iodide
28320-31-2 Downstream products
-
28320-62-9
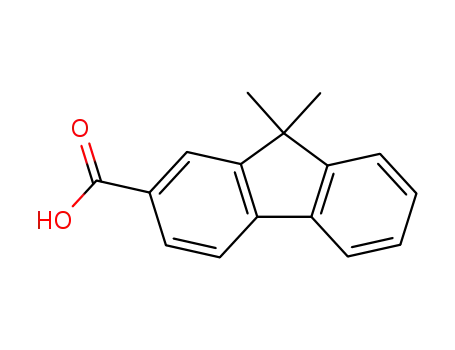
9,9-dimethyl-9H-fluorene-2-carboxylic acid
-
1208005-86-0
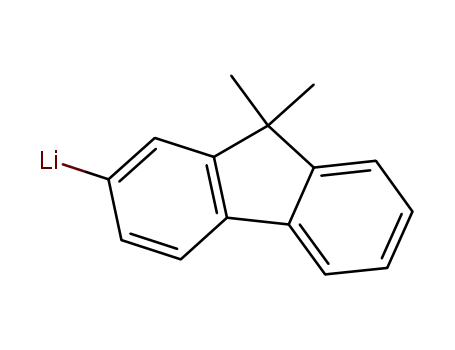
(9,9-dimethylfluoren-2-yl)lithium
-
885684-28-6
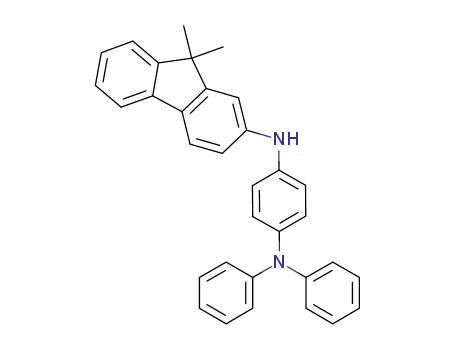
C33H28N2
-
676542-59-9
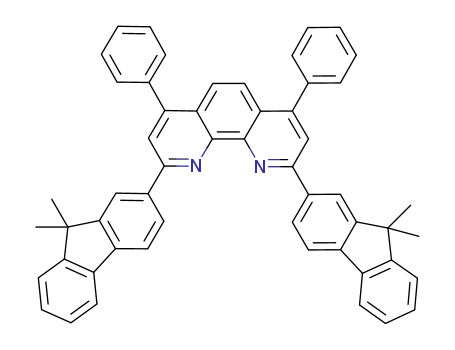
2,9-bis(9,9-dimethyl-9H-fluoren-2-yl)-4,7-diphenyl-1,10-phenanthroline
Relevant Products
-
Phenol,4-bromo-2-chloro- 3964-56-5
CAS:3964-56-5
-
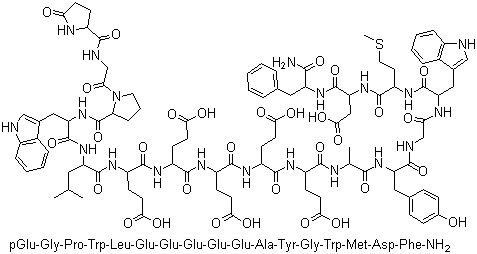
Gastrin I Human
CAS:10047-33-3
-
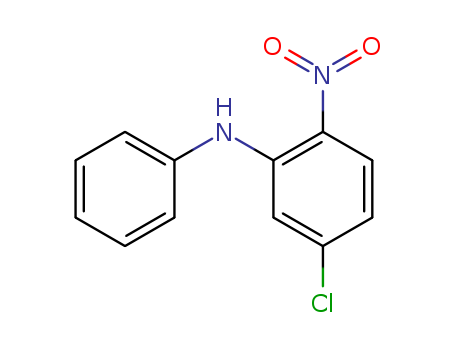
5-Chloro-2-nitrodiphenylamine
CAS:25781-92-4