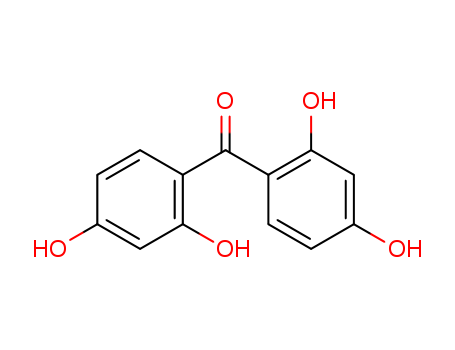
131-55-5
- Product Name:2,2',4,4'-Tetrahydroxybenzophenone
- Molecular Formula:C13H10O5
- Purity:99%
- Molecular Weight:
Product Details
Appearance:yellow to brown crystalline powder
2,2',4,4'-Tetrahydroxybenzophenone Intermediates 131-55-5 Boldenone Undecylenate for sale
- Molecular Formula:C13H10O5
- Molecular Weight:246.219
- Appearance/Colour:yellow to brown crystalline powder
- Melting Point:198-200 °C(lit.)
- Refractive Index:1.4825 (estimate)
- Boiling Point:531.2 °C at 760 mmHg
- PKA:6.98±0.35(Predicted)
- Flash Point:289.2 °C
- PSA:97.99000
- Density:1.526 g/cm3
- LogP:1.74000
2,2',4,4'-Tetrahydroxybenzophenone Intermediates Usage
2,2',4,4'-Tetrahydroxybenzophenone (BZP-2) is water-soluble and light-yellow powder (figure 1). BZP-2 is widely used in perfumes to prevent their degradation due to light. BZP-2 is a UV-absorber that helps protect against product degradation because of UV-light exposure and it is widely used in sunscreens and in cosmetics (figure 2), such as: anti-aging” creams and hair sprays and shampoos, paints and plastics. It is a uV-absorber that helps protect against product degradation because of uV-light exposure. It can also mask odor associated with a formulation’s composition.
Preparation
Obtained (small amount) by melting a fluorescin chloride/sodium hydroxide mixture in the presence of a very few water at 230–240° for 2–3 h.
Contact allergens
BZP-2 is widely used in perfumes to prevent their degradation due to light. It can cause allergic contact dermatitis.
Safety Profile
Moderately toxic by ingestion. Aneye irritant. Mutation data reported. When heated todecomposition it emits toxic fumes of NOx.
131-55-5 Relevant articles
Method for catalytically synthesizing ultraviolet light absorber BP-2
-
Paragraph 0020; 0030-0033; 0038-0049, (2020/11/23)
The invention discloses a method for catalytically synthesizing an ultraviolet light absorber BP-2. The method is high in synthesis yield, simple in production process, high in product purity, low in cost and suitable for industrial production.
Synthetic method of ultraviolet ray absorber BP-2
-
Paragraph 0016; 0027; 0030, (2016/10/10)
The invention discloses a synthetic method of an ultraviolet ray absorber BP-2. The method has the advantages of simple process, mild conditions, low production cost, and suitableness for industrial production.
Synthesis, SAR and biological evaluation of natural and non-natural hydroxylated and prenylated xanthones as antitumor agents
Zhang, Xiaojin,Li, Xiang,Tao, Lei,Gao, Yuan,Gong, Dandan,Xi, Meiyang,Xu, Xiaoli,Guo, Qinglong,You, Qidong,Ye, Suofu,Zhang, Yu,Meng, Huyan,Zhang, Mingqian,Gao, Wenlei
, p. 1012 - 1025,14 (2012/12/12)
The new prenylated xanthone 20 with a relatively simple structure, namely 1,3,8-trihydroxy-2-prenylxanthone, was found to exhibit potent antitumor activities comparable to mangostin against all the five cancer cell lines. Further mechanistic studies suggested that compound 20 induces apoptosis and causes cell cycle arrest at S phase in HepG2 cells. These results have highlighted compound 20 as a new potential lead candidate for future development of novel potent broad-spectrum antitumor agents.
Evaluation of polyhydroxybenzophenones as α-glucosidase inhibitors
Hu, Xuesen,Xiao, Yang,Wu, Jianlong,Ma, Lin
experimental part, p. 71 - 77 (2011/09/21)
This experiment was designed to synthesize 18 kinds of polyhydroxybenzophenones by using Friedel-Crafts reaction, and to measure the inhibitory activity on α-glucosidase with p-nitrophenyl-β-D- galactopyranoside (PNPG) as a substrate. Compound 11 was found to be the most potent inhibitor. Copyright
131-55-5 Process route
-
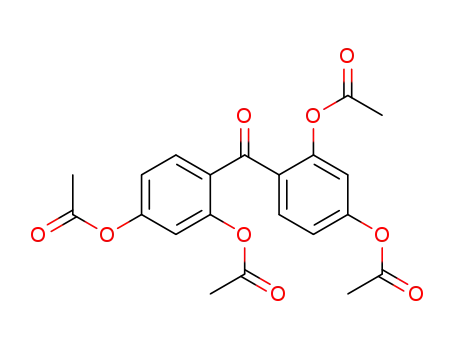
-
carbonylbis(benzene-4,1,3-triyl) tetraacetate

-
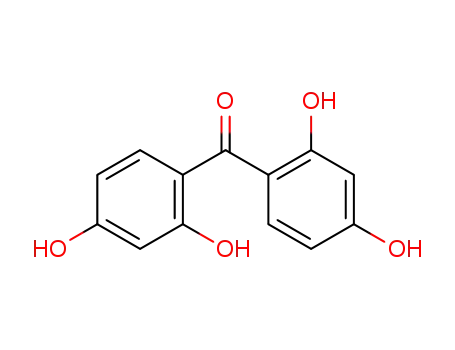
- 131-55-5
2,2',4,4'-tetrahydroxybenzophenone
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 20 - 100 ℃; for 6h; Temperature; Autoclave; Large scale;
|
82.1% |
-
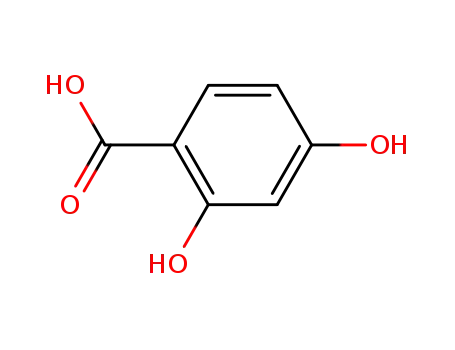
- 89-86-1
4-hydroxysalicylic acid

-
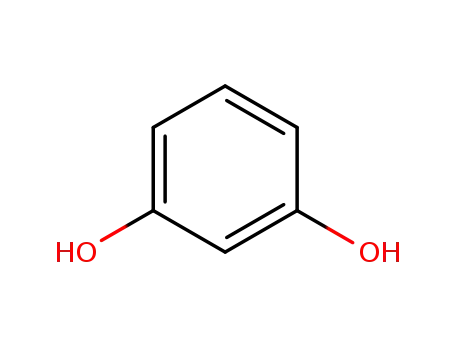
- 108-46-3
recorcinol

-

- 131-55-5
2,2',4,4'-tetrahydroxybenzophenone
| Conditions | Yield |
|---|---|
|
With zinc(II) chloride; trichlorophosphate; at 65 ℃; for 3h; Inert atmosphere;
|
73% |
|
With zinc(II) chloride; trichlorophosphate;
|
|
|
With zinc(II) chloride; at 125 - 140 ℃;
|
|
|
With zinc(II) chloride; trichlorophosphate; at 70 ℃; for 2.5h;
|
|
|
4-hydroxysalicylic acid; recorcinol; With zinc(II) chloride; trichlorophosphate; at 70 ℃; for 2.5h;
With water; at 4 ℃; for 24h; Cooling with ice;
|
|
|
With phosphoric acid; zinc(II) chloride; trichlorophosphate;
|
|
|
With toluene-4-sulfonic acid; In Trichloroethylene; at 105 - 110 ℃; for 2h; Solvent; Reagent/catalyst; Temperature; Large scale;
|
131-55-5 Upstream products
-
151-10-0
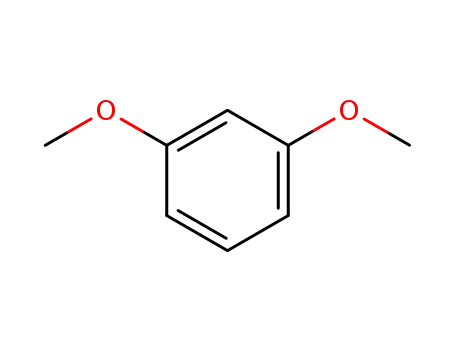
1,3-Dimethoxybenzene
-
89-86-1

4-hydroxysalicylic acid
-
108-46-3

recorcinol
-
131-54-4
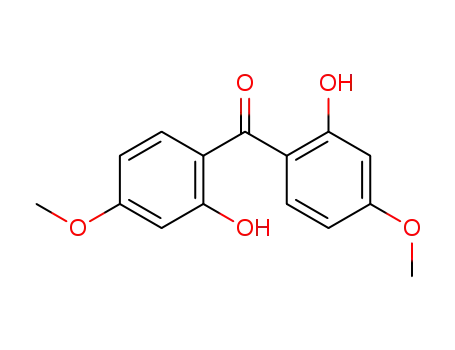
bis(2-hydroxy-4-methoxyphenyl)-methanone
131-55-5 Downstream products
-
1214-24-0
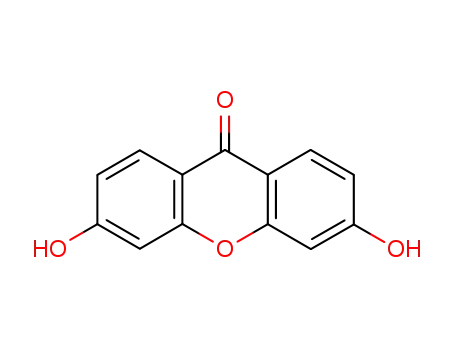
3,6-dihydroxy-xanthen-9-one
-
3555-85-9
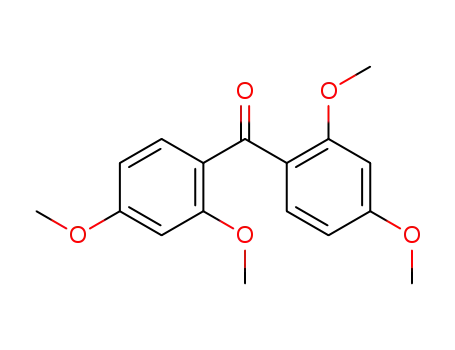
2,2',4,4'-tetramethoxybenzophenone
-
117767-16-5
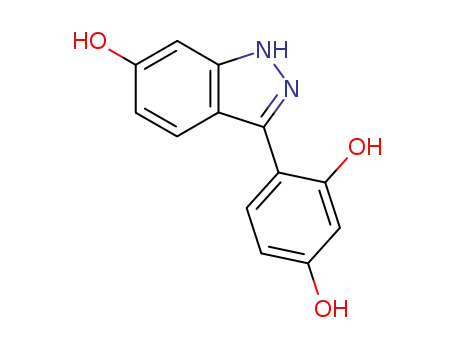
4-(6-hydroxy-1H-indazol-3-yl)-1,3-benzenediol
-
117767-15-4
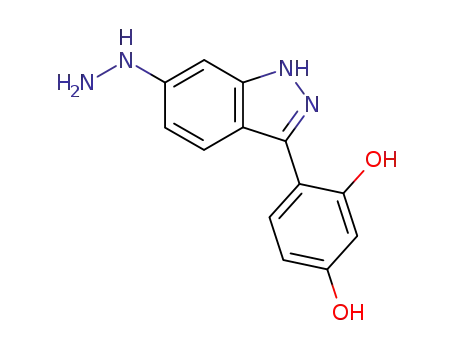
4-(6-hydrazino-1H-indazol-3-yl)-1,3-benzenediol
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
4-Hydroxycinnamic acid
CAS:501-98-4
-
Gastrin I Human
CAS:10047-33-3