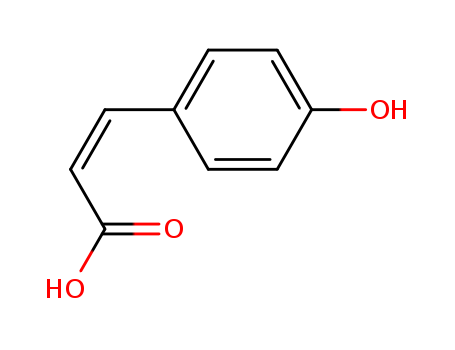
501-98-4
- Product Name:4-Hydroxycinnamic acid
- Molecular Formula:C9H8O3
- Purity:99%
- Molecular Weight:
Product Details
Appearance:Off-white to beige or greenish powder
Factory supply 4-Hydroxycinnamic acid 501-98-4, reasonable price, Good Supplier In China
- Molecular Formula:C9H8O3
- Molecular Weight:164.161
- Appearance/Colour:Off-white to beige or greenish powder
- Vapor Pressure:7.19E-05mmHg at 25°C
- Melting Point:214 °C (dec.)(lit.)
- Refractive Index:1.6
- Boiling Point:346.052 °C at 760 mmHg
- PKA:4.65±0.10(Predicted)
- Flash Point:177.284 °C
- PSA:57.53000
- Density:1.33 g/cm3
- LogP:1.49000
4-Hydroxycinnamic acid 501-98-4 Usage
trans-p-Coumaric Acid is the E-isomer of p-Coumaric Acid (C755365), a hydroxy derivative of Cinnamic Acid with antioxidant properties. p-Coumaric acid is a is a major component of lignocellulose. Off-white to beige or greenish powder. Soluble in alcohol, ether and hot water, slightly soluble in benzene, insoluble in petroleum ether. Stud ies suggest that p-Coumaric Acid may reduce the risk of cancer by reducing the formation of carcinogenic nitrosamines.
Definition
ChEBI: p-coumaric acid is the trans-isomer of 4-coumaric acid. 4-coumaric acid is a coumaric acid in which the hydroxy substituent is located at C-4 of the phenyl ring. It has a role as a plant metabolite. It is a conjugate acid of a 4-coumarate.
Purification Methods
Crystallise p-coumaric acid from H2O (charcoal). It forms needles from concentrated aqueous solutions as the anhydrous acid, but from hot dilute solutions the monohydrate acid separates on slow cooling. The acid (33g) has been crystallised from 2.5L of H2O (1.5g charcoal) yielding 28.4g of recrystallised acid, m 207o. It is insoluble in *C6H6 or pet ether. The UV in 95% EtOH has max 223 and 286nm ( 14,450 and 19000 M-1cm-1). [UV Wheeler & Covarrubias J Org Chem 28 2015 1963, Corti Helv Chim Acta 32 681 1949, Beilstein 10 IV 1005.]
InChI:InChI=1/C9H8O3/c1-6(9(11)12)7-2-4-8(10)5-3-7/h2-5,10H,1H2,(H,11,12)
501-98-4 Relevant articles
Understanding the role played by protic ionic liquids (PILs) and the substituent effect for enhancing the generation of: Z-cinnamic acid derivatives
Rodríguez, Roció B.,Rodríguez, Roció B.,Zapata, Ramiro L.,Salum, Mariá L.,Salum, Mariá L.,Erra-Balsells, Rosa,Erra-Balsells, Rosa
, p. 819 - 830 (2020/07/03)
Photoisomerization of a series of substituted E-cinnamic acids in MeCN in their acid forms and as their corresponding protic ionic liquids (PILs) with light of 300 nm is studied. Thus, understanding of these fundamental aspects has allowed us to establish an excellent and practical synthetic protocol for successfully synthesizing Z-cinnamic acids. This journal is
Four New Flavonoids Isolated from the Aerial Parts of Cadaba rotundifolia Forssk. (Qadab)
Al-Hamoud, Gadah Abdulaziz,Orfali, Raha Saud,Sugimoto, Sachiko,Yamano, Yoshi,Alothyqi, Nafee,Alzahrani, Ali Mohammed,Matsunami, Katsuyoshi
, (2019/06/21)
Cadaba rotundifolia (Forssk.) (family: Capparaceae; common name: Qadab) is one of four species that grow in the Red Sea costal region in the Kingdom of Saudi Arabia.
Flavonoid glycosides from Sedum bulbiferum
Warashina, Tsutomu,Miyase, Toshio
, p. 1199 - 1204 (2017/12/26)
The MeOH extract from dried whole Sedum bulbiferum MAKINO (Crassulaceae) plants yielded 34 compounds, including six new flavonoid glycosides and 28 known compounds.
501-98-4 Process route
-
![peonidin 3-O-[2-O-(2-O-(trans-feruloyl)-glucosyl)-6-O-(cis-p-coumaroyl)-glucoside]-5-O-[6-O-(malonyl)-glucoside]](/upload/2023/1/e7f4c5c8-f2e6-4f4a-b363-bea6413f154d.png)
-
peonidin 3-O-[2-O-(2-O-(trans-feruloyl)-glucosyl)-6-O-(cis-p-coumaroyl)-glucoside]-5-O-[6-O-(malonyl)-glucoside]

-
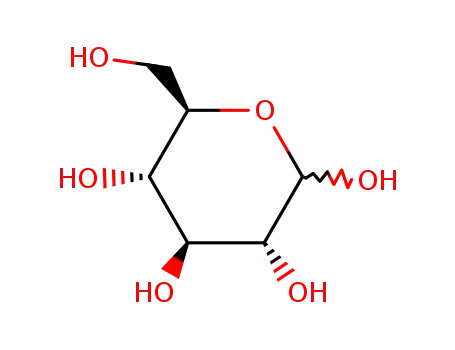
- 2280-44-6
D-Glucose

-
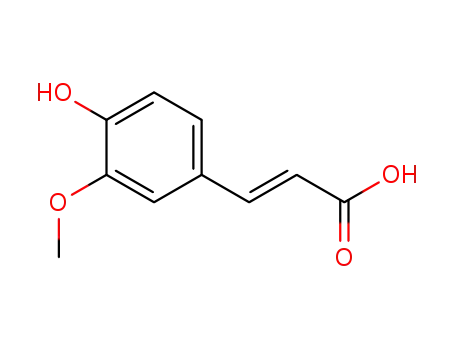
- 1135-24-6,537-98-4
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
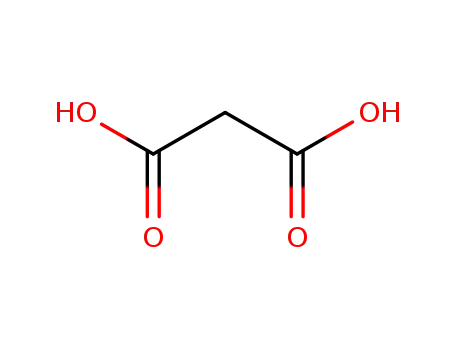
- 141-82-2
malonic acid

-
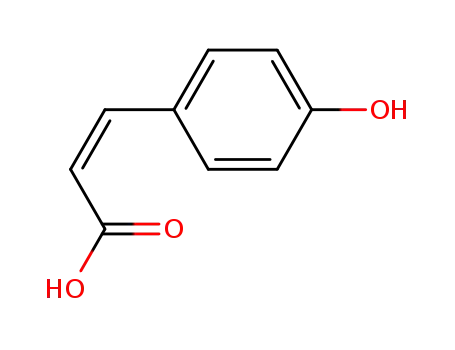
- 501-98-4,4501-31-9,7400-08-0,50940-26-6
(Z)-p-coumaric acid

-
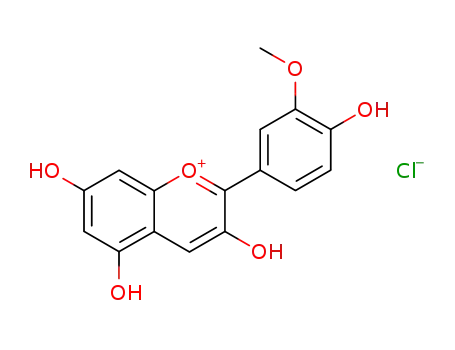
- 134-01-0
peonidin chloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; water; at 100 ℃; for 1h;
|
-
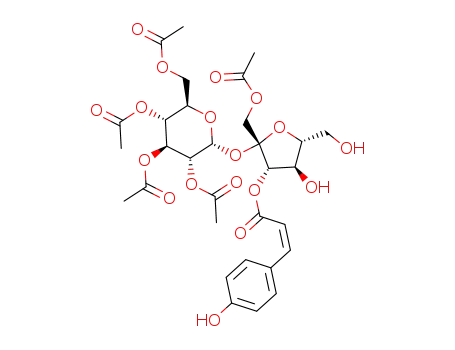
- 1392307-47-9
1,2',3',4',6'-penta-O-acetyl-3-O-(Z)-p-coumaroylsucrose

-
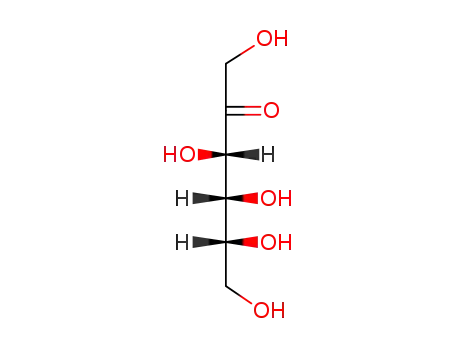
- 57-48-7,18875-34-8
D-Fructose

-
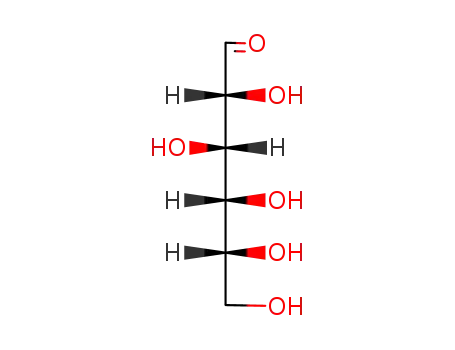
- 50-99-7
D-glucose

-

- 501-98-4,4501-31-9,7400-08-0,50940-26-6
(Z)-p-coumaric acid
| Conditions | Yield |
|---|---|
|
With sulfuric acid; water; In 1,4-dioxane; at 90 ℃; for 3h;
|
501-98-4 Upstream products
-
7400-08-0
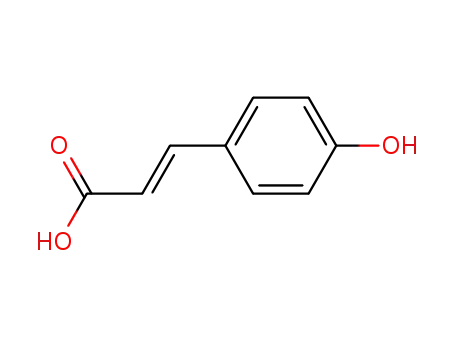
p-Coumaric Acid
-
29588-94-1
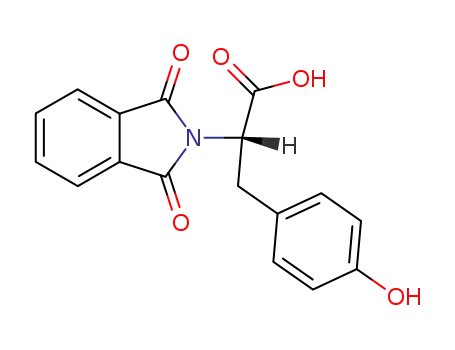
N-phthaloyl-L-tyrosine
-
3943-97-3
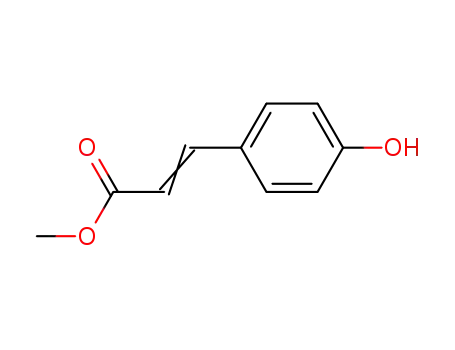
methyl p-hydroxycinnamate
-
1225064-68-5
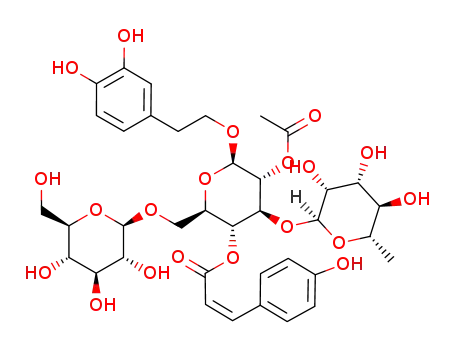
kankanoside H2
501-98-4 Downstream products
-
123-07-9
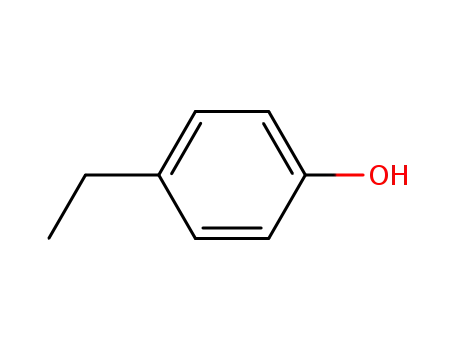
4-Ethylphenol
-
672341-24-1
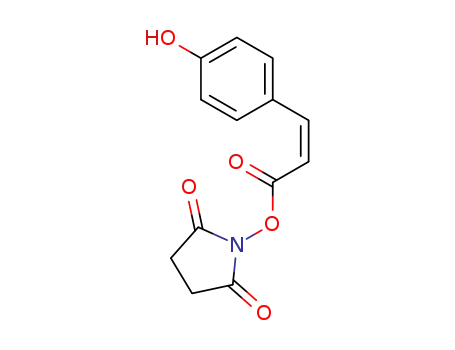
cis-p-coumaroylsuccinimide
-
632323-91-2
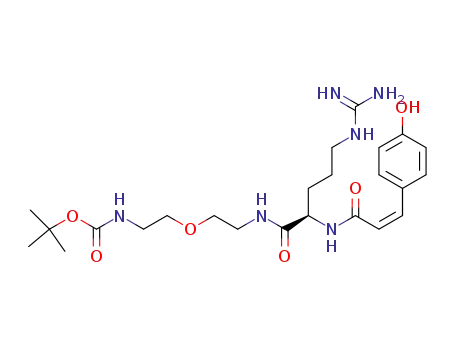
N-(2-(tert-butoxycarbonylamino)ethoxyethyl) Nα-cis-p-coumaroyl-D-arginine amide
-
632323-92-3
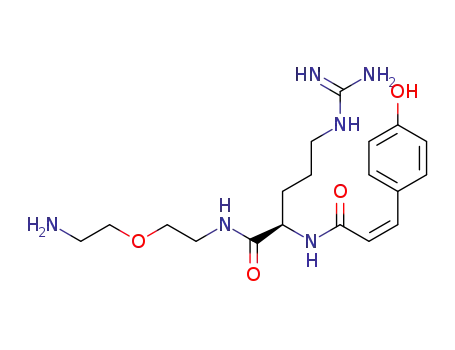
(R)-5-Guanidino-2-[(Z)-3-(4-hydroxy-phenyl)-acryloylamino]-pentanoic acid [2-(2-amino-ethoxy)-ethyl]-amide
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
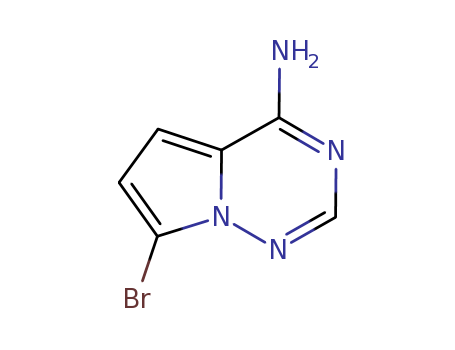
7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine
CAS:937046-98-5
-
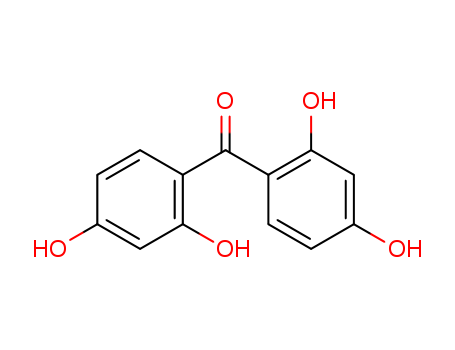
2,2',4,4'-Tetrahydroxybenzophenone
CAS:131-55-5