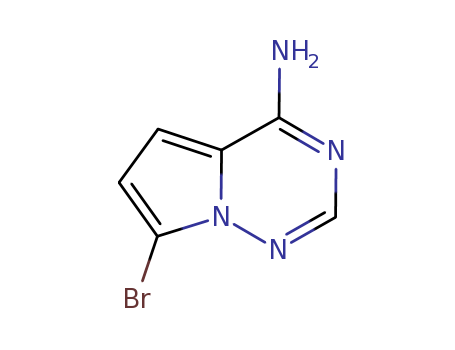
937046-98-5
- Product Name:7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine
- Molecular Formula:C6H5BrN4
- Purity:99%
- Molecular Weight:
Product Details
High Purity 7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine Wholesaler, 937046-98-5 for sale
- Molecular Formula:C6H5BrN4
- Molecular Weight:213.037
- PKA:2.82±0.30(Predicted)
- PSA:56.21000
- Density:2.098 g/cm3
- LogP:1.65520
7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine 937046-98-5 Usage
7-bromopyrrolo[2,1-f][1,2,4]triazin-4-amine is used in preparation of N-Heterocyclic compounds for pharmaceutical use including as anticancer agents and PRMT5 inhibitors.
InChI:InChI=1S/C6H5BrN4/c7-5-2-1-4-6(8)9-3-10-11(4)5/h1-3H,(H2,8,9,10)
937046-98-5 Relevant articles
Identification of a potent, selective, and efficacious Phosphatidylinositol 3-kinase δ (PI3Kδ) inhibitor for the treatment of immunological disorders
Qingjie Liu†, Qing Shi†, David Marcoux*†Orcid, Douglas G. Batt†, Lyndon Cornelius†, Lan-Ying Qin†, Zheming Ruan†, James Neels†, Myra Beaudoin-Bertrand†, Anurag S. Srivastava†, Ling Li†, Robert J. Cherney†, Hua Gong†, Scott H. Watterson†, Carolyn Weigelt†, Kathleen M. Gillooly†, Kim W. McIntyre†, Jenny H. Xie†, Mary T. Obermeier†, Aberra Fura†, Bogdan Sleczka†, Kevin Stefanski†, R. M. Fancher†, Shweta Padmanabhan‡, Thatipamula RP‡, Ipsit Kundu‡, Kallem Rajareddy§, Rodney Smith†, James K. Hennan†, Dezhi Xing†, Jingsong Fan†, Paul C. Levesque†, Qian Ruan†, Sidney Pitt†, Rosemary Zhang†, Donna Pedicord†, Jie Pan†, Melissa Yarde†, Hao Lu†, Jonathan Lippy†, Christine Goldstine†, Stacey Skala†, Richard A. Rampulla†, Arvind Mathur†, Anuradha Gupta‡, Pirama Nayagam Arunachalam‡, John S. Sack†, Jodi K. Muckelbauer†, Mary Ellen Cvijic†, Luisa M. Salter-Cid†, Rajeev S. Bhide‡, Michael A. Poss†, John Hynes†, Percy H. Carter†, John E. Macor∥, Stefan Ruepp†, Gary L. Schieven†, and Joseph A. Tino†
J. Med. Chem. 2017, 60, 12, 5193–5208
SO 4 and concentrated under reduced pressure yielding 7-bromopyrrolo[2,1-f][1,2,4]triazin-4-amine and 7-bromo-5-fluoropyrrolo[2,1-f][1,2,4]triazin-4-amine. To the resulting solid and …
Diastereoselective Flexible Synthesis of Carbocyclic C-Nucleosides
Lukáš Maier†‡⊥, Prashant Khirsariya†‡⊥, Ondřej Hylse†‡, Santosh Kumar Adla†, Lenka Černová†, Michal Poljak†, Soňa Krajčovičová†, Erik Weis†, Stanislav Drápela§, Karel Souček‡§, and Kamil Paruch*†‡
J. Org. Chem. 2017, 82, 7, 3382–3402
7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine as well as the coupling were in our hands more reliable than with the (known) unprotected analog. (15) The SEM groups were removed from …
Discovery of 7-(3-(piperazin-1-yl)phenyl)pyrrolo[2,1-f][1,2,4]triazin-4-amine derivatives as highly potent and selective PI3Kδ inhibitors
《Bioorganic & Medicinal Chemistry Letters》 LY Qin,Z Ruan,RJ Cherney,TGM Dhar,J Neels,CA Weigelt,JS Sack,AS Srivastava,LAM Cornelius,JA Tino
(2017)
As demonstrated in preclinical animal models, the disruption of PI3Kδ expression or its activity leads to a decrease in inflammatory and immune responses. Therefore, inhibition of PI3Kδ may provide an alternative treatment for autoimmune diseases, such as RA, SLE, and respiratory ailments. Herein, we disclose the identification of 7-(3-(piperazin-1-yl)phenyl)pyrrolo[2,1-f][1,2,4]triazin-4-amine derivatives as highly potent, selective and orally bioavailable PI3Kδ inhibitors.
937046-98-5 Process route
-
![pyrrolo[2,1-f][1,2,4]triazin-4-amine](/upload/2023/1/7a8c09ee-7cbe-4f29-9f18-f130325e3b2f.png)
- 159326-68-8
pyrrolo[2,1-f][1,2,4]triazin-4-amine

-
![7-bromo-pyrrolo[2,1-f][1,2,4]triazin-4-ylamine](/upload/2023/1/83f240c7-859e-430f-9341-e37d2dc5411d.png)
- 937046-98-5
7-bromo-pyrrolo[2,1-f][1,2,4]triazin-4-ylamine
| Conditions | Yield |
|---|---|
|
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; at -15 - -10 ℃; for 0.75h; Large scale;
|
91% |
|
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In N,N-dimethyl-formamide; at -40 - 0 ℃;
|
91% |
|
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In N,N-dimethyl-formamide; at -20 ℃; for 1.5h;
|
90% |
|
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In N,N-dimethyl-formamide; at -20 ℃; for 1.5h;
|
90% |
|
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In N,N-dimethyl-formamide; at -20 ℃; for 1.5h; regioselective reaction;
|
77% |
|
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In N,N-dimethyl-formamide; at -20 ℃; for 1h;
|
75% |
|
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In N,N-dimethyl-formamide; at -40 - 0 ℃; for 0.75h;
|
-
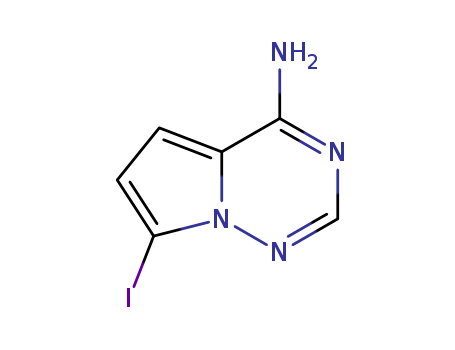
![7-iodopyrrolo-[2,1-f][1,2,4]-triazin-4-amine](/upload/2023/1/c083bf67-68fc-40ae-b60d-c4fbff59d63a.png)
- 1770840-43-1
7-iodopyrrolo-[2,1-f][1,2,4]-triazin-4-amine

-
![7-bromo-pyrrolo[2,1-f][1,2,4]triazin-4-ylamine](/upload/2023/1/83f240c7-859e-430f-9341-e37d2dc5411d.png)
- 937046-98-5
7-bromo-pyrrolo[2,1-f][1,2,4]triazin-4-ylamine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: chloro-trimethyl-silane / tetrahydrofuran / 0.17 h / 20 °C
1.2: 0.02 h / 0 °C
2.1: 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / N,N-dimethyl-formamide / -40 - 0 °C
With chloro-trimethyl-silane; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In tetrahydrofuran; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1.1: chloro-trimethyl-silane / tetrahydrofuran / 20 °C / Flow reactor
1.2: 20 °C / Flow reactor
1.3: 20 °C / Flow reactor
2.1: 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / N,N-dimethyl-formamide / -40 - 0 °C
With chloro-trimethyl-silane; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In tetrahydrofuran; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1.1: chloro-trimethyl-silane; phenylmagnesium chloride / tetrahydrofuran / 20 °C / Flow reactor
1.2: 20 °C / Flow reactor
1.3: 20 °C / Flow reactor
2.1: 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione / N,N-dimethyl-formamide / -40 - 0 °C
With chloro-trimethyl-silane; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; phenylmagnesium chloride; In tetrahydrofuran; N,N-dimethyl-formamide;
|
937046-98-5 Upstream products
-
159326-68-8
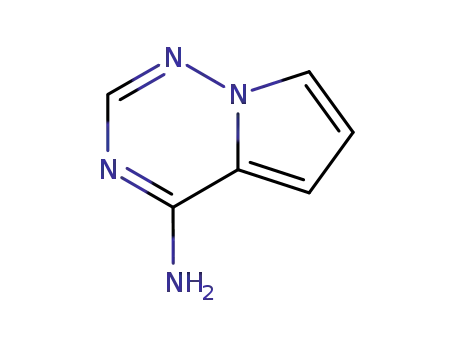
pyrrolo[2,1-f][1,2,4]triazin-4-amine
-
937046-96-3
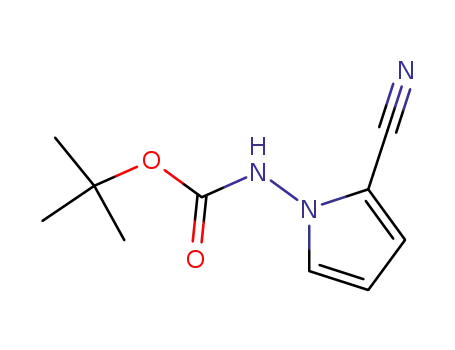
tert-butyl (2-cyano-1H-pyrrol-1-yl)carbamate
-
1770840-43-1
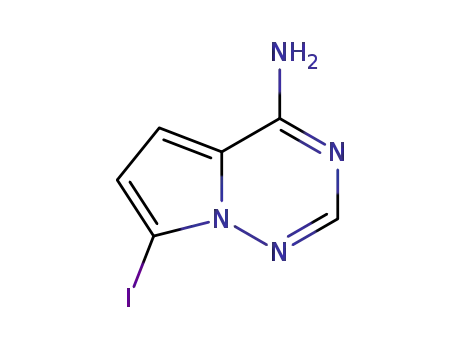
7-iodopyrrolo-[2,1-f][1,2,4]-triazin-4-amine
937046-98-5 Downstream products
-
937048-34-5
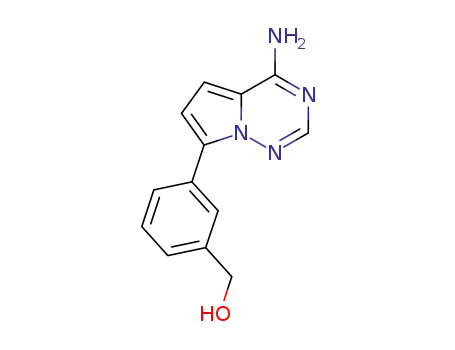
[3-(4-amino-pyrrolo[2,1-f][1,2,4]triazin-7-yl)-phenyl]-methanol
-
937048-40-3
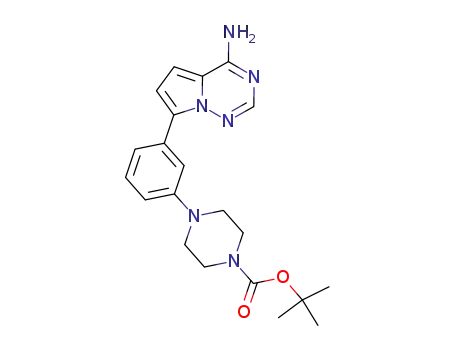
4-[3-(4-amino-pyrrolo[2,1-f][1,2,4]triazin-7-yl)-phenyl]-piperazine-1-carboxylic acid tert-butyl ester
-
937048-10-7
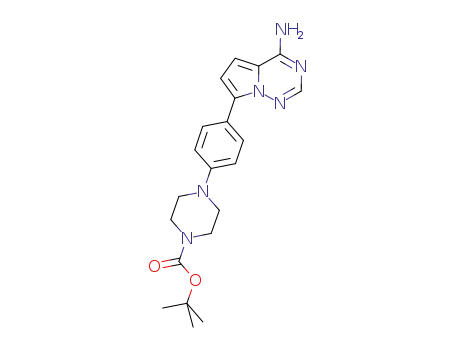
tert-butyl 4-[4-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)phenyl]piperazine-1-carboxylate
-
937048-01-6
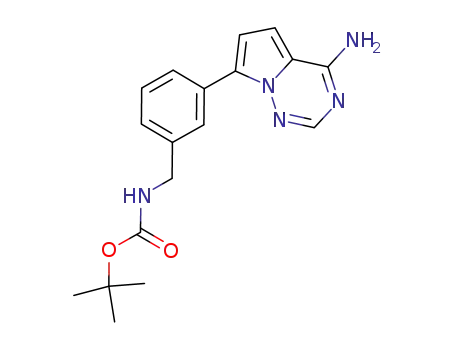
[3-(4-amino-pyrrolo[2,1-f][1,2,4]triazin-7-yl)-benzyl]-carbamic acidtert-butyl ester
Relevant Products
-
2-Bromonicotinic acid 35905-85-2
CAS:35905-85-2
-
Remdesivir intermediate
CAS:1770840-43-1
-
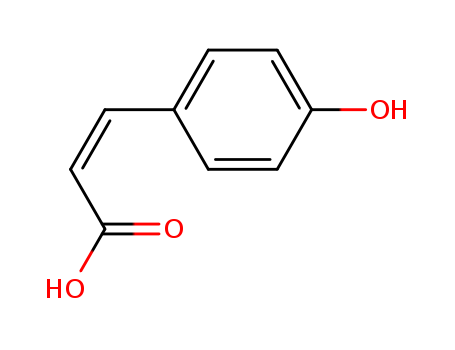
4-Hydroxycinnamic acid
CAS:501-98-4