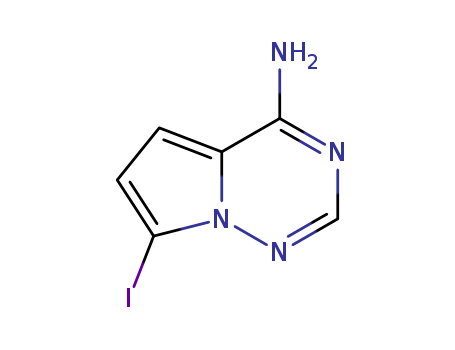
1770840-43-1
- Product Name:Remdesivir intermediate
- Molecular Formula:
- Purity:99%
- Molecular Weight:
Product Details
Best quality Remdesivir intermediate 1770840-43-1 for sale, in bulk supply
- Molecular Formula:C6H5IN4
- Molecular Weight:260.037
Remdesivir intermediate 1770840-43-1 Usage
Remdesivir, an investigational broad-spectrum antiviral drug produced by Gilead Sciences Inc. Remdesivir intermediate 1770840-43-1 is a novel antiviral drug in the class of nucleotide analogs and can be used as an intermediate for APIs. Remdesivir, an intravenous nucleotide prodrug, has been approved for treating COVID-19 in hospitalized adults and pediatric patients.
Remdesivir has been found with broad-spectrum anti-RNA virus activity via inhibiting viral replication through premature termination of RNA transcription [[1], [2], [3]].
1770840-43-1 Relevant articles
Modified Synthesis and Isolation of an Advanced Intermediate of Remdesivir
Mohite, Dhanaji M.; Pandey, Arjun P.; Chavhan, Pandurang M.
Current Organic Chemistry, Volume 26, Number 23, 2022, pp. 2143-2150(8)
Synthesis and isolation of an advanced intermediate (S)-2-Ethylbutyl 2-(((S)-(4- nitrophenoxy) (phenoxy) phosphoryl) amino) propanoate (1b), which is being used for the manufacture of the prodrug diastereoisomer 1d called Remdesivir have been carried out in high yield with efficient stereoselectivity.
Preparation method and application of remdesivir intermediate
-
Paragraph 0051-0074, (2021/07/24)
The invention discloses a preparation method and application of a remdesivir intermediate. DMF is not used as a solvent, so that the risk that genotoxic impurities are easily generated by DMF is reduced, the storage safety problem of DMF is reduced, and the production cost is reduced.
Human carboxylesterase 1A plays a predominant role in the hydrolytic activation of remdesivir in humans
Feng Zhang , Hong-Xin Li , Tian-Tian Zhang , Yuan Xiong , Hao-Nan Wang , Zhen-Hui Lu , Lei Xiong , Yu-Qi He , Guang-Bo Ge
Chemico-Biological Interactions Volume 351, 5 January 2022, 109744
All these studies will provide crucial information for deciphering the key enzyme(s) responsible for the first step of remdesivir hydrolysis, which will be very useful for predicting inter-individual variability in response to remdesivir and for guiding the rational use of this anti-COVID-19 agent in clinical settings.
1770840-43-1 Process route
-
![pyrrolo[2,1-f][1,2,4]triazin-4-amine](/upload/2023/1/a8a2d476-1ca5-4e2c-aff8-6a2776769e54.png)
- 159326-68-8
pyrrolo[2,1-f][1,2,4]triazin-4-amine

-
![7-iodopyrrolo-[2,1-f][1,2,4]-triazin-4-amine](/upload/2023/1/0f2ea73a-1ef9-48e7-a49f-c82122d187e1.png)
- 1770840-43-1
7-iodopyrrolo-[2,1-f][1,2,4]-triazin-4-amine
| Conditions | Yield |
|---|---|
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 0 ℃; for 1h;
|
95% |
|
pyrrolo[2,1-f][1,2,4]triazin-4-amine; With Iodine monochloride; In N,N-dimethyl-formamide; at -25 - -10 ℃; for 3h; Inert atmosphere;
With N-iodo-succinimide; acetic acid; In N,N-dimethyl-formamide; at -10 ℃; for 1h; Reagent/catalyst; Solvent; Inert atmosphere;
|
90% |
|
pyrrolo[2,1-f][1,2,4]triazin-4-amine; With pyridine; iodine; In ethyl acetate; at 0 - 10 ℃; for 1h;
With dihydrogen peroxide; In ethyl acetate; at 20 ℃; for 10h;
|
85% |
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 0 ℃; for 3h;
|
81% |
|
With iodine; urea hydrogen peroxide adduct; In ethanol; at 78 ℃; for 1h; Reagent/catalyst; Solvent; Temperature;
|
80% |
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 0 ℃; for 2h;
|
77% |
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 3 - 8 ℃; for 4h; Large scale;
|
76.1% |
|
With N-iodo-succinimide; In dimethyl sulfoxide; at 20 ℃; for 16h;
|
75% |
|
With N-iodo-succinimide; potassium carbonate; In ethanol; at 15 - 30 ℃; for 24h; Large scale;
|
70.8% |
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 0 ℃; for 5h;
|
47% |
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 20 ℃; for 1.5h;
|
|
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 0 ℃; for 3h;
|
|
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 0 ℃; for 3h;
|
|
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
|
|
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 0 ℃; for 3h;
|
|
|
With iodine; potassium carbonate; In N,N-dimethyl-formamide; at 50 ℃; for 2h;
|
|
|
With N-iodo-succinimide; N,N-dimethyl-formamide; at 0 ℃; for 3h;
|
30 g |
-
![4-chloro-7-iodopyrrolo[1,2-f][1,2,4]triazine](/upload/2023/1/5c9e081c-f12d-476c-ba20-1355715cba42.png)
-
4-chloro-7-iodopyrrolo[1,2-f][1,2,4]triazine

-
![7-iodopyrrolo-[2,1-f][1,2,4]-triazin-4-amine](/upload/2023/1/0f2ea73a-1ef9-48e7-a49f-c82122d187e1.png)
- 1770840-43-1
7-iodopyrrolo-[2,1-f][1,2,4]-triazin-4-amine
| Conditions | Yield |
|---|---|
|
With ammonia; In tetrahydrofuran; methanol; at 20 - 55 ℃; for 18h;
|
81% |
1770840-43-1 Upstream products
-
159326-68-8
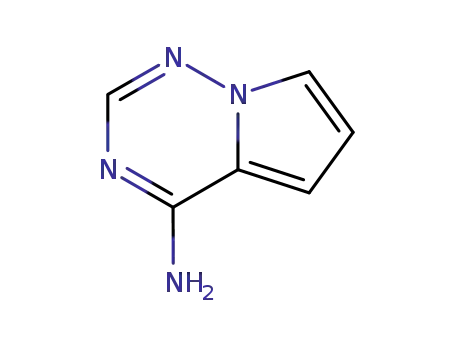
pyrrolo[2,1-f][1,2,4]triazin-4-amine
-
1003-29-8
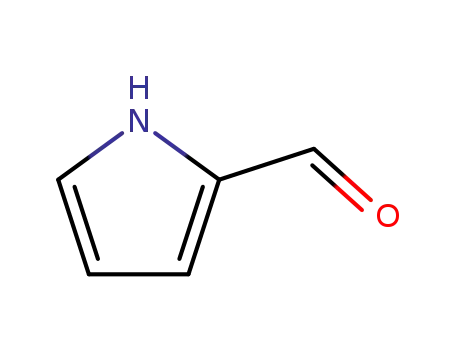
2-pyrrole aldehyde
-
4513-94-4
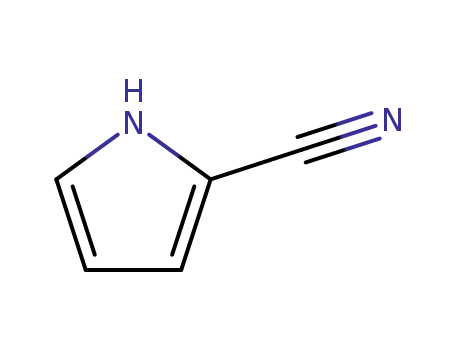
1H-pyrrole-2-carbonitrile
-
159326-71-3
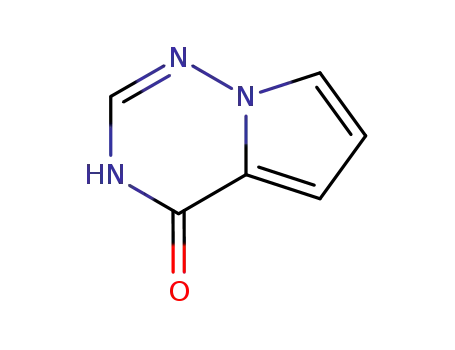
pyrrolo<2,1-f><1,2,4>triazin-4(3H)-one
1770840-43-1 Downstream products
-
158227-80-6
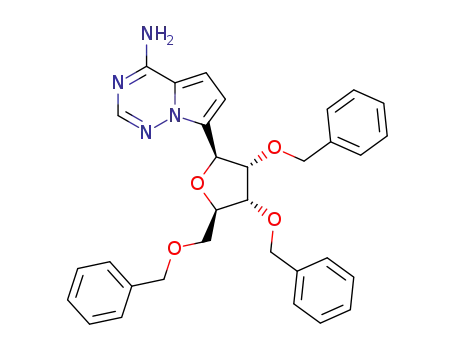
7-((2S,3S,4R,5R)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)-tetrahydrofuran-2-yl)pyrrolo[2,1-f][1,2,4]triazin-4-amine
Relevant Products
-
3-Azaspiro[5.5]undecane,hydrochloride (1:1)
CAS:1125-01-5
-
Sodium dehydroacetate 4418-26-2
CAS:4418-26-2
-
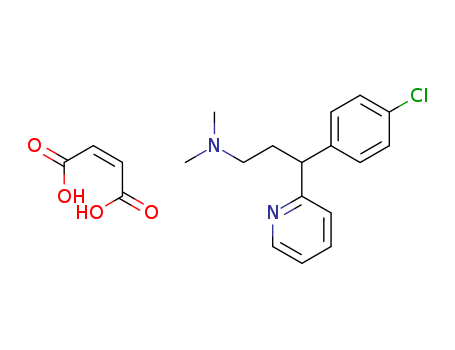
Chlorpheniramine maleate
CAS:113-92-8
-
7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine
CAS:937046-98-5