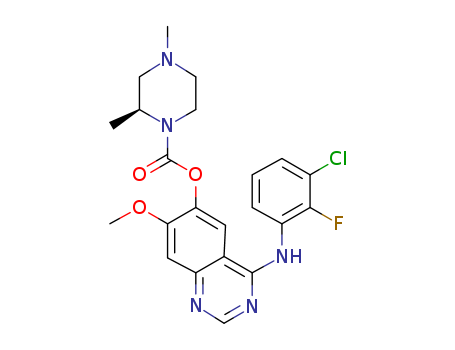
1626387-80-1
- Product Name:AZD 3759
- Molecular Formula:C22H23ClFN5O3
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white to off-white solid
Factory sells AZD3759 1626387-80-1 in bulk supply with best price
- Molecular Formula:C22H23ClFN5O3
- Molecular Weight:459.908
- Appearance/Colour:white to off-white solid
- Melting Point:192.4 °C(alkyl acetate or alcohol), 193.3 °C (water)
- Boiling Point:571.4±50.0 °C(Predicted)
- PKA:6.71±0.40(Predicted)
- PSA:79.82000
- Density:1.358±0.06 g/cm3(Predicted)
- LogP:4.25800
AZD3759 Usage
AZD3759 is a selective EGFR inhibitor that can fully penetrate the blood-brain barrier (BBB), with equal free concentrations in the blood, cerebrospinal fluid, and brain tissue. AZD 3759 is EGFR inhibitor. It is a potent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor.
1626387-80-1 Relevant articles
Preparation method of lung cancer drug AZD3759
-
, (2022/01/20)
The present invention provides a method for preparing a lung cancer drug AZD3759. Using compounds of formula VIII and compounds of formula V. as raw materials, after hydrolysis reaction, condensation reaction, ammonization reaction, acidolysis reaction, methylation reaction, compound of formula I AZD3759 was obtained. The method has a novel route, simple response, environmental friendliness and high yield, which is suitable for industrial large-scale production.
Preparation method of novel anti-cancer drug AZD3759
-
, (2020/09/30)
The invention provides a preparation method of a novel anticancer drug AZD3759, and relates to the technical field of medicinal chemistry. The preparation method provided by the invention has the advantages of cheap and accessible raw materials and lower cost, sodium cyanoborohydride is not used in the reaction process, so that the method is more environment-friendly, the product yield is high, and industrial large-scale production is facilitated.
Novel method of synthetic process of lung cancer targeted compound AZD-3759
-
, (2019/04/09)
The novel method of the synthetic process of the lung cancer targeted compound AZD-3759 has the beneficial effects thatthe raw materials for the novel method are easily obtained, the price is low, the yield is high, the cost is low, the method is environmentally friendly, and industrialized enlarged production can berealized.
1626387-80-1 Process route
-
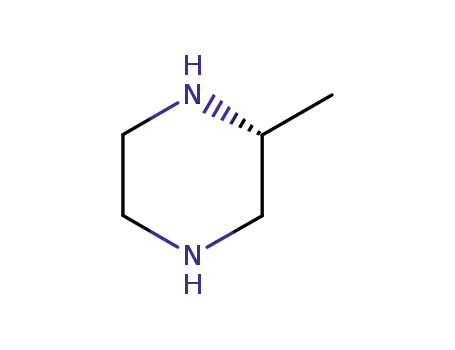
- 75336-86-6
(2R)-methylpiperazine

-
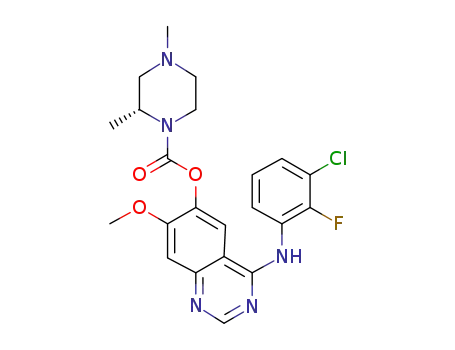
- 1626387-80-1
(R)-4-((3-chloro-2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl-2,4-dimethylpiperazinyl-1-carboxylic acid ester
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: dichloromethane / 1 h / 0 °C
2: pyridine / dichloromethane / 24 h / 0 - 20 °C
3: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 24 h / 0 - 20 °C
4: hydrogenchloride; methanol / water; 1,4-dioxane / 12 h / 20 °C
5: formic acid / water / 3 h / 90 - 100 °C
With pyridine; hydrogenchloride; methanol; formic acid; N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 6 steps
1: dichloromethane / 1 h / 0 °C
2: pyridine / dichloromethane / 24 h / 0 - 20 °C
3: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 24 h / 0 - 20 °C
4: hydrogenchloride / 1,4-dioxane / 12 h / 20 °C
5: formic acid / water / 3 h / 95 °C
6: isopropyl alcohol / 3 h / Reflux
With pyridine; hydrogenchloride; formic acid; N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; dichloromethane; water; N,N-dimethyl-formamide; isopropyl alcohol;
|
|
|
Multi-step reaction with 7 steps
1.1: dichloromethane / 1 h / 0 °C
2.1: pyridine / dichloromethane / 24 h / 0 - 20 °C
3.1: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 24 h / 0 - 20 °C
4.1: hydrogenchloride; methanol / 1,4-dioxane / 12 h / 20 °C
5.1: formic acid / water / 3 h / 95 °C
6.1: N-ethyl-N,N-diisopropylamine / toluene / 0.5 h / 70 °C
6.2: 3 h / 95 °C
7.1: isopropyl alcohol / 3 h / Reflux
With pyridine; hydrogenchloride; methanol; formic acid; N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; dichloromethane; water; N,N-dimethyl-formamide; isopropyl alcohol; toluene;
|
-
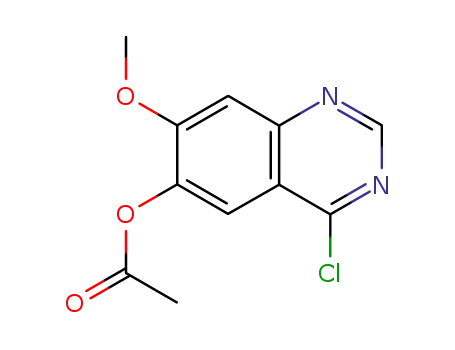
- 230955-75-6
6-acetoxy-4-chloro-7-methoxyquinazoline

-

- 1626387-80-1
(R)-4-((3-chloro-2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl-2,4-dimethylpiperazinyl-1-carboxylic acid ester
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: acetonitrile / 80 °C
2: potassium carbonate; methanol / 20 °C
3: potassium carbonate / N,N-dimethyl-formamide / 20 °C
4: hydrogenchloride / dichloromethane; 1,4-dioxane / 0.5 h / 20 °C
5: sodium cyanoborohydride / methanol / 20 °C
With hydrogenchloride; methanol; sodium cyanoborohydride; potassium carbonate; In 1,4-dioxane; methanol; dichloromethane; N,N-dimethyl-formamide; acetonitrile;
|
|
|
Multi-step reaction with 5 steps
1: acetonitrile / 80 °C
2: potassium carbonate / methanol / 20 °C
3: potassium carbonate / N,N-dimethyl-formamide / 20 °C
4: hydrogenchloride / dichloromethane; 1,4-dioxane / 0.5 h / 20 °C
5: sodium cyanoborohydride / methanol / 20 °C
With hydrogenchloride; sodium cyanoborohydride; potassium carbonate; In 1,4-dioxane; methanol; dichloromethane; N,N-dimethyl-formamide; acetonitrile;
|
|
|
Multi-step reaction with 5 steps
1: acetonitrile / 4 h / Reflux
2: potassium carbonate / methanol / 2 h / 10 - 15 °C
3: potassium carbonate / N,N-dimethyl-formamide / 25 °C
4: hydrogenchloride / methanol; water; 1,4-dioxane / 1 h / 25 °C
5: sodium cyanoborohydride / methanol / 25 °C
With hydrogenchloride; sodium cyanoborohydride; potassium carbonate; In 1,4-dioxane; methanol; water; N,N-dimethyl-formamide; acetonitrile;
|
|
|
Multi-step reaction with 5 steps
1: isopropyl alcohol / 3 h / Reflux
2: ammonium hydroxide; methanol / 6 h / 20 - 30 °C
3: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 24 h / 0 - 20 °C
4: hydrogenchloride; methanol / water; 1,4-dioxane / 12 h / 20 °C
5: formic acid / water / 3 h / 90 - 100 °C
With hydrogenchloride; methanol; formic acid; ammonium hydroxide; N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; water; N,N-dimethyl-formamide; isopropyl alcohol;
|
|
|
Multi-step reaction with 5 steps
1: ammonium hydroxide / methanol / 6 h / Reflux
2: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 24 h / 0 - 20 °C
3: hydrogenchloride / 1,4-dioxane / 12 h / 20 °C
4: formic acid / water / 3 h / 95 °C
5: isopropyl alcohol / 3 h / Reflux
With hydrogenchloride; ammonium hydroxide; formic acid; N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; methanol; water; N,N-dimethyl-formamide; isopropyl alcohol;
|
|
|
Multi-step reaction with 5 steps
1: hydrogenchloride / water / 9 h / 90 °C
2: dicyclohexyl-carbodiimide; dmap / tetrahydrofuran / 6 h / 40 °C
3: triethylamine / isopropyl alcohol / 3 h / Reflux
4: hydrogenchloride / water; ethyl acetate / 2 h / 20 °C
5: formic acid / water / 3 h / 100 °C
With hydrogenchloride; dmap; formic acid; triethylamine; dicyclohexyl-carbodiimide; In tetrahydrofuran; water; ethyl acetate; isopropyl alcohol;
|
1626387-80-1 Upstream products
-
179688-52-9
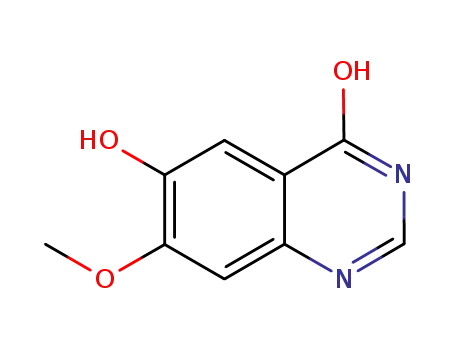
4,6-dihydroxy-7-methoxyquinazoline
-
179688-53-0
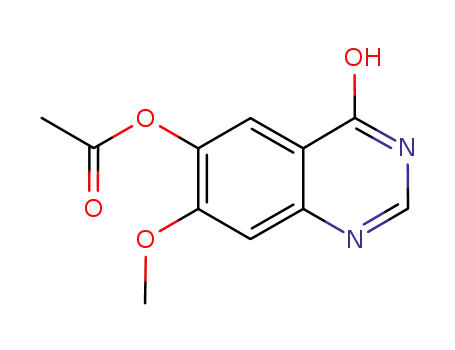
(4-hydroxy-7-methoxyquinazolin-6-yl) acetate
-
230955-75-6

6-acetoxy-4-chloro-7-methoxyquinazoline
-
740081-22-5
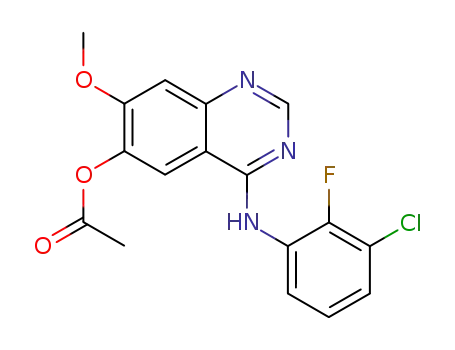
4-[(3-chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl acetate
1626387-80-1 Downstream products
-
1626387-81-2
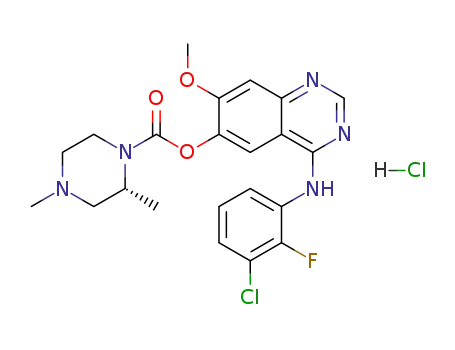
4-[(3-chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl (2R)-2,4-dimethylpiperazine-1-carboxylate hydrochloride
Relevant Products
-
Metformin
CAS:657-24-9
-
D-Mannitol
CAS:69-65-8