15307-79-6
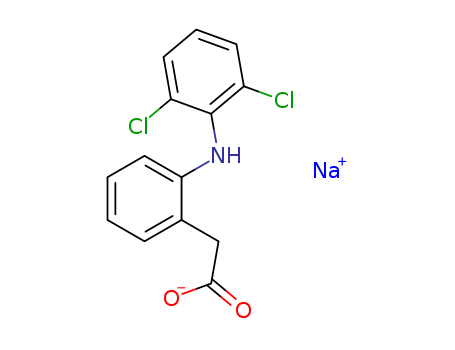
- Product Name:Diclofenac sodium
- Molecular Formula:C14H10Cl2NNaO2
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white or off-white powder
Hot Sale, top purity 15307-79-6 Diclofenac sodium for sale
- Molecular Formula:C14H10Cl2NNaO2
- Molecular Weight:318.135
- Appearance/Colour:white or off-white powder
- Melting Point:288-290 °C
- Boiling Point:412 °C at 760 mmHg
- PKA:4(at 25℃)
- Flash Point:203 °C
- PSA:52.16000
- Density:0.781 g/cm3
- LogP:3.10240
Diclofenac sodium Usage
Diclofenac Sodium is the sodium salt form of diclofenac, a benzene acetic acid derivate and nonsteroidal anti-inflammatory drug (NSAID) with analgesic, antipyretic and anti-inflammatory activity. It is a phenylacetic acid derivative belonging to the class of the non-selective non-steroidal anti-inflammatory drugs (NSAIDs). It exhibits analgesic, antipyretic and anti-inflammatory activity. Due to its poor solubility, the parenteral formulation of diclofenac sodium (Voltarol ampoules) currently available in Europe contains the solvents propylene glycol and benzyl alcohol that allows intramuscular and intravenous administration. Diclofenac sodium has long been used to treat acute pain and inflammation, and is effective in various acute forms of pain.
Definition
ChEBI: Diclofenac sodium is the sodium salt of diclofenac. It contains a diclofenac(1-).
InChI:InChI=1/C12H9Cl2N.C2H4O2.Na/c13-10-7-4-8-11(14)12(10)15-9-5-2-1-3-6-9;1-2(3)4;/h1-8,15H;1H3,(H,3,4);/q;;+1/p-1
15307-79-6 Relevant articles
Synthesis method of diclofenac sodium
-
Paragraph 0024; 0027; 0028; 0031; 0032; 0035, (2021/09/21)
The invention discloses a synthesis method of diclofenac sodium.The synthesis method is stable, easy to operate, low in cost, high in yield and suitable for industrial production.
Synthesis process of diclofenac sodium
-
Paragraph 0084-0087, (2021/09/26)
The invention provides a synthesis process of diclofenac sodium, which is obtained by acylation reaction of o-aminobenzene acetate with 2 and 6 - dichlorophenoxy acetic acid respectively and acylation with chlorobenzoyl chloride followed by nucleophilic substitution with 2, 6 - dichlorophenol or 2 and 6 -dichlorophenol. Is hydrolyzed to give sodium diclofenac sodium. The synthesis process is simplified, the reaction condition is mild, and the yield and industrial popularization and application are facilitated.
Synthetic method of diclofenac sodium
-
Paragraph 0017-0026, (2019/04/26)
The method for synthesizing diclofenac sodium according to the invention has a purity of more than 98% and a total yield of two steps of up to 90% or more. According to the synthetic method of the diclofenac sodium, the purity of the product is up tomore than 98%, the total yield of two steps is up to more than 90%, and the yield is high; and D-glucosamine hydrochloride is adopted to replace ligands such as 8-hydroxyquinoline and the like which are high in price and large in environmental pollution, the pollution to the environment is reduced while the production cost is reduced.
15307-79-6 Process route
-
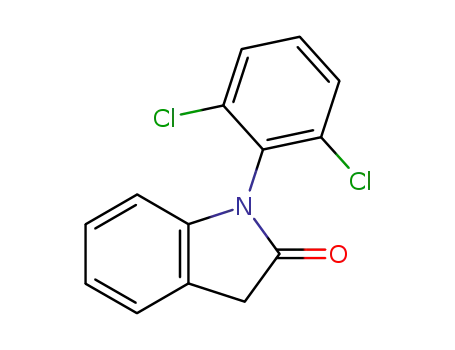
- 15362-40-0
1-(2,6-dichlorophenyl)indolin-2-one

-
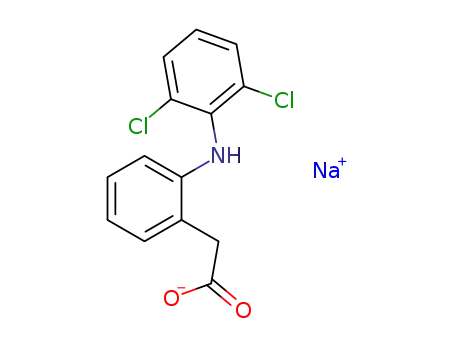
- 15307-79-6
diclofenac sodium
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In ethanol; water; for 4h; Heating;
|
93% |
|
With sodium dithionite; water; sodium hydroxide; for 6h; Reflux;
|
92.3% |
|
With sodium hydroxide; In ethanol; for 3h; Heating;
|
85% |
|
With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide; In toluene; for 10h; Reflux;
|
83.6% |
|
With sodium hydroxide; for 2h; Temperature; Reflux;
|
4.035 g |
|
With sodium hydroxide; at 90 ℃; for 6h; Temperature;
|
-
![2-[(2,6-dichlorophenyl)amino]phenylacetic acid methyl ester](/upload/2023/1/b7514fec-9eb2-4f48-8162-02328c19eea7.png)
- 15307-78-5
2-[(2,6-dichlorophenyl)amino]phenylacetic acid methyl ester

-

- 15307-79-6
diclofenac sodium
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; N,N-dimethyl-formamide; at 80 ℃; for 24h;
|
96.1% |
15307-79-6 Upstream products
-
15362-40-0

1-(2,6-dichlorophenyl)indolin-2-one
-
66156-75-0
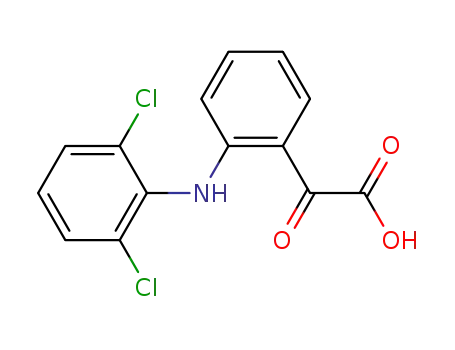
2-(2-((2,6-dichlorophenyl)amino)phenyl)-2-oxoacetic acid
-
100754-92-5
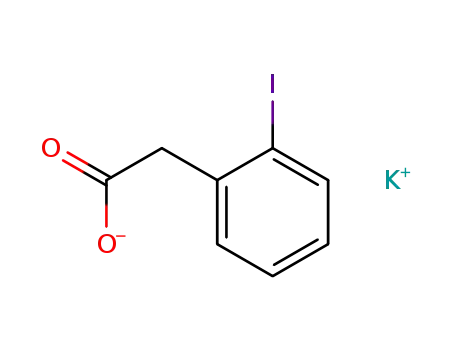
potassium 2-iodophenylacetate
-
608-31-1
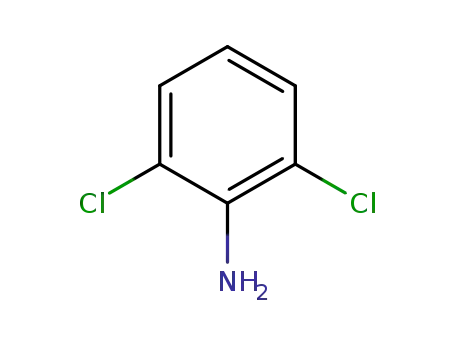
2,6-Dichloroaniline
15307-79-6 Downstream products
-
174316-61-1
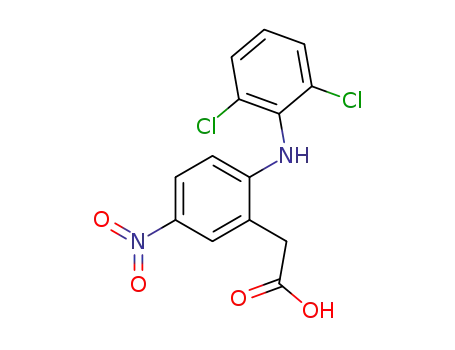
2-(2,6-dichloroanilino)-5-nitrophenylacetic acid
-
15362-40-0

1-(2,6-dichlorophenyl)indolin-2-one
-
22121-58-0
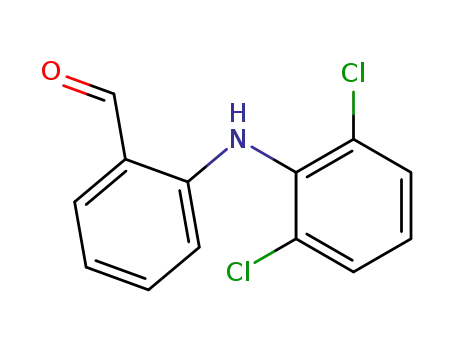
2-[(2,6-dichlorophenyl)amino]benzaldehyde
-
15307-78-5
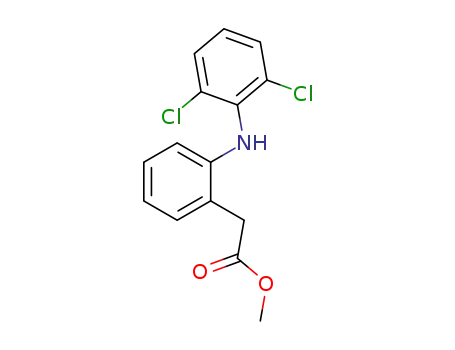
2-[(2,6-dichlorophenyl)amino]phenylacetic acid methyl ester
Relevant Products
-
Tianeptine sodium salt
CAS:30123-17-2
-
D-Biotin
CAS:58-85-5
-
Progesterone
CAS:57-83-0