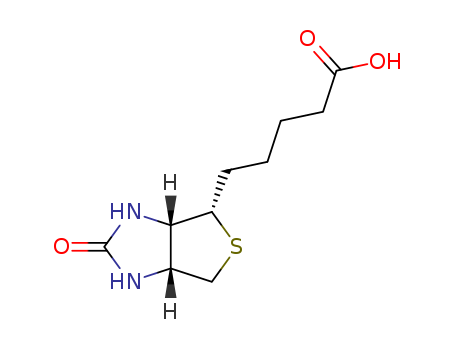
Product Details
Appearance:white powder
D-BIOTIN 58-85-5 for sale, in bulk supply, reasonable price
- Molecular Formula:C10H16N2O3S
- Molecular Weight:244.315
- Appearance/Colour:white powder
- Vapor Pressure:1.19E-14mmHg at 25°C
- Melting Point:231-233 °C(lit.)
- Refractive Index:90.5 ° (C=2, 0.1mol/L NaOH)
- Boiling Point:573.6 °C at 760 mmHg
- PKA:4.74±0.10(Predicted)
- Flash Point:300.7 °C
- PSA:103.73000
- Density:1.267 g/cm3
- LogP:1.45440
D-BIOTIN Usage
D-Biotin, also called Vitamin H, is a colorless, water-soluble member of the group of B-vitamins. Formerly it was known as vitamin H or coenzyme R. D-biotin (which is usually referred to as biotin) exhibits biological activity. Biotin is widely distributed in animals and plants, and the natural presence of biotin is mainly in the form of binding with other molecules. D-Biotin is a clear, white crystalline powder. This product is very soluble in water and in ethanol, and practically insoluble in organic solvents. D-biotin is also used in skin-conditioning agents. As a feed additive, it is mainly used for poultry and sow feed. Usually the premixed mass fraction is 1%-2%. It is nutritional supplement.
According to China GB2760-90 regulations, it could be used as a food industry as a processing aid. It has physiological functions to prevent skin diseases and promote lipid metabolism and so on. The vitamin Biotin improves the quality of keratin structures in cosmetic applications and hence has a positive effect on fine and brittle hair and nails. The biotin content of cancerous tumors is higher than that of normal tissue.
Side effect
Bursts of cystic acne of jaw and chin are the most common side effects of biotin. The specific reason is not very clear. And usually this symptom will disappear by itself after a few weeks.
Definition
ChEBI: An organic heterobicyclic compound that consists of 2-oxohexahydro-1H-thieno[3,4-d]imidazole having a valeric acid substituent attached to the tetrahydrothiophene ring. The parent of the class of biotins.
Safety Profile
An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx and SOx.
InChI:InChI=1/C10H16N2O3S/c13-8(14)4-2-1-3-7-9-6(5-16-7)11-10(15)12-9/h6-7,9H,1-5H2,(H,13,14)(H2,11,12,15)/p-1/t6-,7-,9+/m0/s1
58-85-5 Relevant articles
METHOD FOR PRODUCING BIOTIN
-
Paragraph 0067-0083, (2021/02/25)
To provide a production method for obtaining high-purity biotin at good yields.SOLUTION: A method for producing biotin includes a process in which a composition comprising biotin represented by the formula (I) is brought into contact with hydrosulfite salt, before the biotin is extracted.SELECTED DRAWING: None
Synthesis of a Key Intermediate for D-Biotin via 1,3Cycloaddition of a Thiocarbonyl Ylide and Sila-Pummerer Rearrangement
T Yamano,M Tanaka,K Takanohashi
Heterocycles 1993
ChemInform is a weeklying Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInformof an article which was published elsewhere, please select a "Full Text" option. The original article is trackable via the "References" option.
Selective modification of sulfamidate-containing peptides
Busto, Jesús H.,Jiménez-Osés, Gonzalo,Mazo, Nuria,Navo, Claudio D.,Peregrina, Jesús M.
supporting information, p. 6265 - 6275 (2020/09/07)
Hybrid peptides whose N-terminal residues are activated in the form of α-methylisoserine-derived cyclic sulfamidates exhibit rich reactivity as electrophiles, allowing site- and stereoselective modifications at different backbone and side chain positions.
58-85-5 Process route
-
-
C19H24N2O3S

-
- 58-85-5,22879-79-4
biotin
| Conditions | Yield |
|---|---|
|
C19H24N2O3S; With palladium on activated charcoal; hydrogen; In ethanol; at 80 - 85 ℃; for 15h; under 11400.8 - 15201 Torr;
With sodium hydroxide; In water; at 25 - 30 ℃; for 5h; Solvent;
|
86.9% |
-
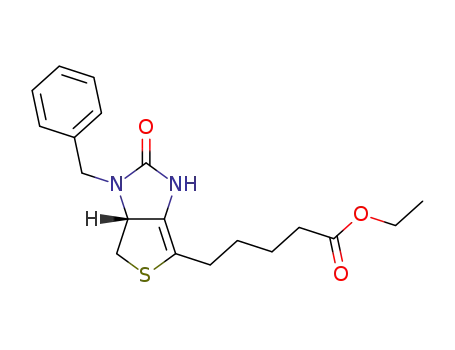
![5-((3aR,6S,6aS)-1-Benzyl-2-oxo-hexahydro-thieno[3,4-d]imidazol-6-yl)-pentanoic acid](/upload/2023/1/960f9b92-a683-472f-b7e6-588dbfd745a9.png)
-
5-((3aR,6S,6aS)-1-Benzyl-2-oxo-hexahydro-thieno[3,4-d]imidazol-6-yl)-pentanoic acid

-

- 58-85-5,22879-79-4
biotin
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; sodium dithionite; water; sodium hydroxide; In water; at 55 - 90 ℃; for 0.5h; Reagent/catalyst;
|
95.8% |
58-85-5 Upstream products
-
75-44-5
phosgene
-
22342-46-7
(2S,3S,4R)-cis-5-(3,4-diaminotetrahydro-2-thienyl)valeric acid
-
58-85-5
(+/-)-biotin
-
76335-62-1
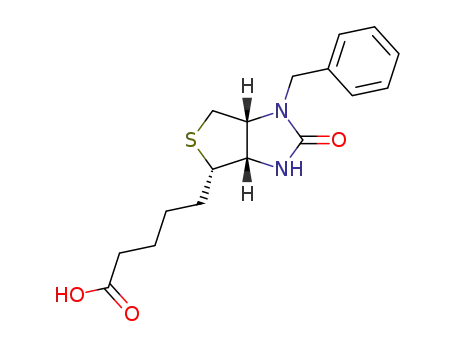
5-[(3aS)-1( oder !3)-benzyl-2-oxo-(3ar,6ac)-hexahydro-thieno[3,4-d]imidazol-4t-yl]-valeric acid
58-85-5 Downstream products
-
608-16-2
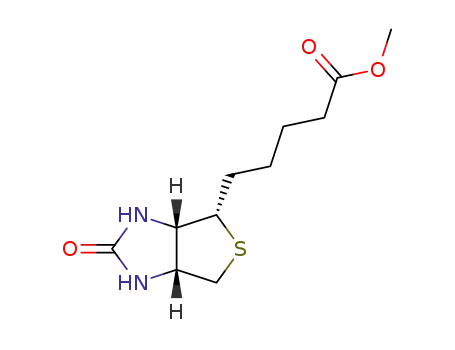
biotin methyl ester
-
120550-35-8
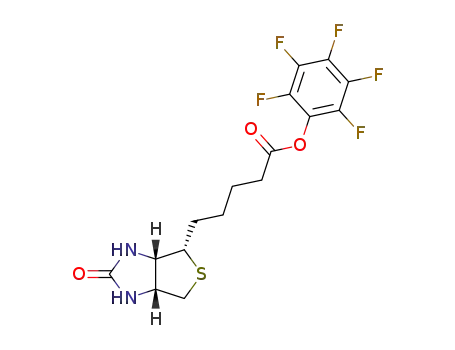
biotin pentafluorophenyl ester
-
6706-15-6
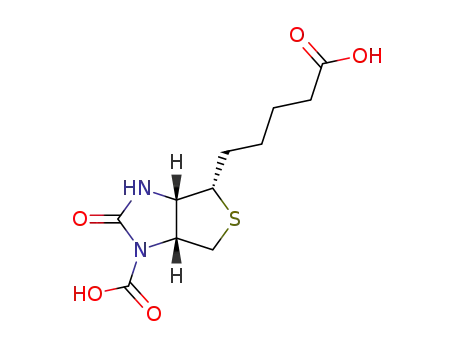
carboxybiotin
-
16968-98-2
bisnorbiotin
Relevant Products
-
Sparteine sulfate anhydrous
CAS:299-39-8
-
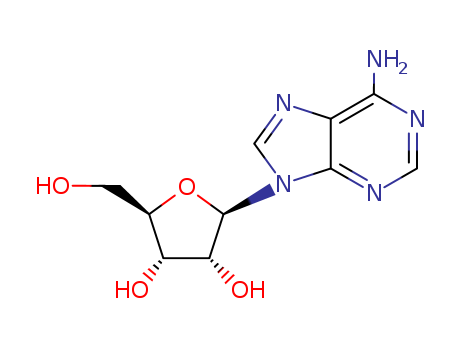
Adenosine
CAS:58-61-7
-
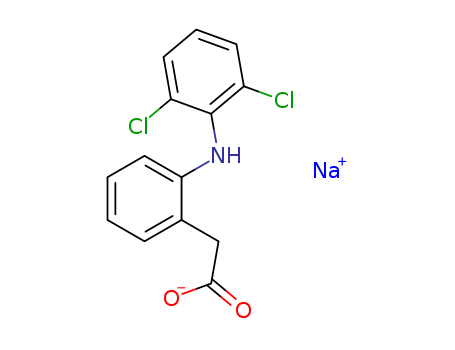
Diclofenac sodium
CAS:15307-79-6