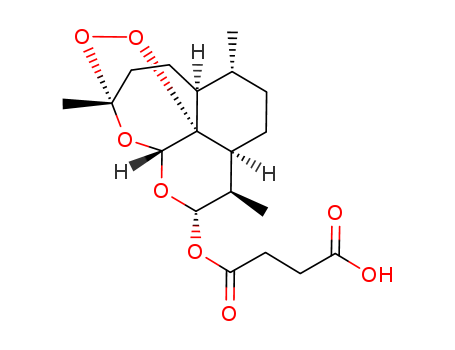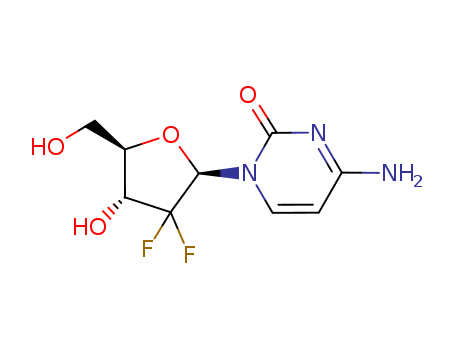
95058-81-4
- Product Name:Gemcitabine
- Molecular Formula:C9H11F2N3O4
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white powder
95058-81-4 Wholesaler, Gemcitabine in stock for sale
- Molecular Formula:C9H11F2N3O4
- Molecular Weight:263.201
- Appearance/Colour:white powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:168.64 °C
- Refractive Index:1.652
- Boiling Point:482.7 °C at 760 mmHg
- PKA:11.65±0.70(Predicted)
- Flash Point:245.7 °C
- PSA:110.60000
- Density:1.84 g/cm3
- LogP:-0.70740
Gemcitabine 95058-81-4 Usage
Gemcitabine(Gemzar), a pyrimidine antimetabolite, is a cornerstone in PDA treatment belongs to the group of medicines called antimetabolites, It is an anticancer nucleoside analog that inhibits the growth of HL-60 promyelocytic leukemia cells with an LC50 value of 40 nM. It inhibits the growth of MX-1 mammary, CX-1, HC-1, GC3, and VRC5 colon, LX-1, Calu-6, and NCI-H460 lung, and HS766T, PaCa-2, PANC-1, and BxPC-3 pancreatic cancer tumors in mouse xenograft models (45-93% inhibition). Gemcitabine is a nucleoside analog and a chemotherapeutic agent, It is used for breast cancer treatment. First-line treatment for locally advanced pancreatic cancer.
Indications
Gemcitabine is the gold standard drug for pancreatic cancer treatment, which is an antimetabolite ranking among the most prescribed anticancer drugs worldwide. The drug is available as the hydrochloride salt in 200- and1,000-mg lyophilized single-dose vials for IV use. It is the single most active agent for the treatment of metastatic pancreatic cancer, and it is used as a first-line treatment for both pancreatic and small cell lung cancer. It is administered by intravenous infusion. The dose-limiting toxicity is bone marrow suppression.
Hazard
Human systemic effects
InChI:InChI=1/C9H11F2N3O4/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17)/t4-,6-,7?/m1/s1
95058-81-4 Relevant articles
Barriers and Opportunities for gemcitabine in pancreatic cancer therapy
Alica K. Beutel and Christopher J. Halbrook
American Journal of Physiology, 10 FEB 2023
As compared with other cytotoxic chemotherapies, gemcitabine is generally considered a tolerable compound for the majority of patients with PDA. Gemcitabine is transported into cells by hENT1, hENT2, hCNT1, hCNT2, and hCNT3, while most of the uptake is mediated through hENT1 (23, 24).
microRNAs Associated with Gemcitabine Resistance via EMT, TME, and Drug Metabolism in Pancreatic Cancer
Naotake Funamizu *,Masahiko Honjo,Kei Tamura,Katsunori Sakamoto,Kohei Ogawa andYasutsugu Takada
Cancers 2023, 15(4), 1230
This novel evidence of gemcitabine resistance will drive further research to elucidate the mechanisms of chemoresistance and improve patient outcomes. Compared with 5-fluorouracil, gemcitabine was found to be superior in relieving symptoms caused by progression in patients with pancreatic cancer.
Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial
Prof Robin Kate Kelley, MD * Makoto Ueno, MD * Prof Changhoon Yoo, MD Prof Richard S Finn, MD Prof Junji Furuse, MD Prof Zhenggang Ren, MD Thomas Yau, MD Heinz-Josef Klümpen, PhD Prof Stephen L Chan, MD Masato Ozaka, MD Prof Chris Verslype, MD Mohamed Bou
The Lancet, 2023
Based on a statistically significant, clinically meaningful improvement in overall survival compared with gemcitabine and cisplatin without any new safety signals, pembrolizumab plus gemcitabine and cisplatin could be a new treatment option for patients.
95058-81-4 Process route
-
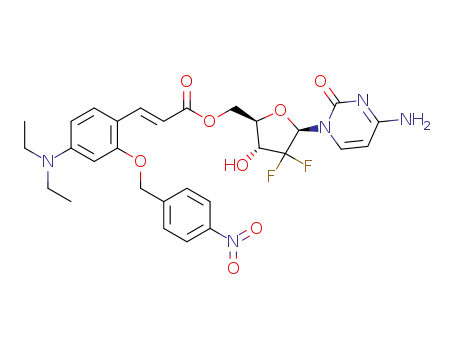
-
C29H31F2N5O8

-
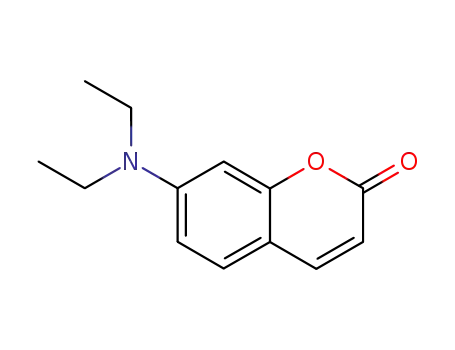
- 20571-42-0
7-diethylamino-2H-benzopyran-2-one

-
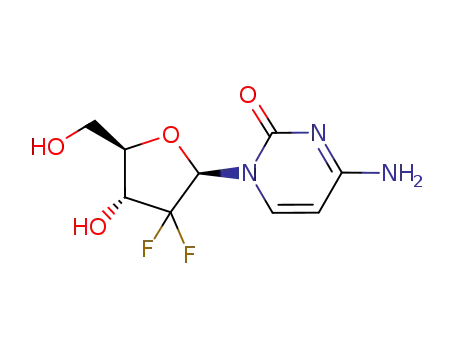
- 95058-81-4,103882-85-5
gemcitabine
| Conditions | Yield |
|---|---|
|
With nitroreductase; β-nicotinamide adenine dinucleotide, disodium salt, reduced form; In aq. phosphate buffer; dimethyl sulfoxide; at 37 ℃; for 0.5h; Time; Inert atmosphere; UV-irradiation;
|
-
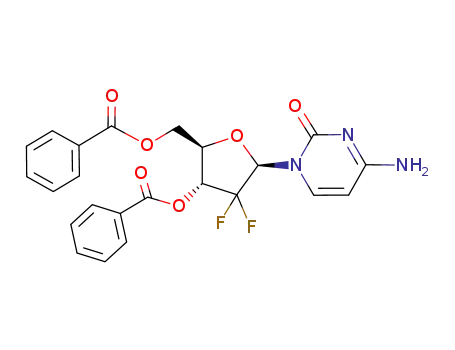
- 134790-39-9,1268237-46-2
(2'-deoxy-2',2'-difluorocytidine)-3',5'-dibenzoate

-

- 95058-81-4,103882-85-5
gemcitabine
| Conditions | Yield |
|---|---|
|
With ammonium hydroxide; In methanol; at 20 ℃; for 3h;
|
82.7% |
|
With sodium t-butanolate; In methanol; at 20 ℃; for 2h;
|
95058-81-4 Upstream products
-
18027-23-1
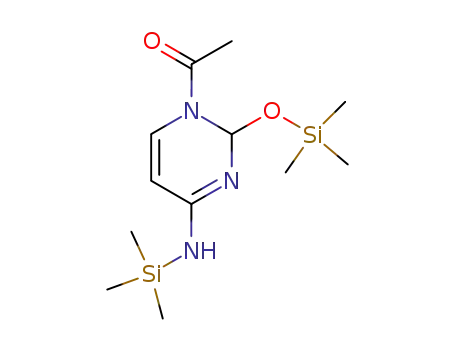
bis(trimethylsilyl)-N-acetylcytosine
-
103882-89-9
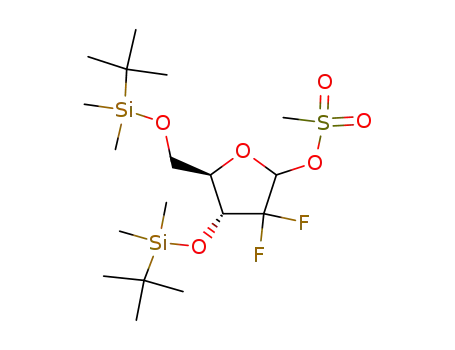
3,5-bis-O-(tert-butyldimethylsilyl)-1-O-(methanesulfonyl)-2-deoxy-2,2-difluororibose
-
952408-92-3
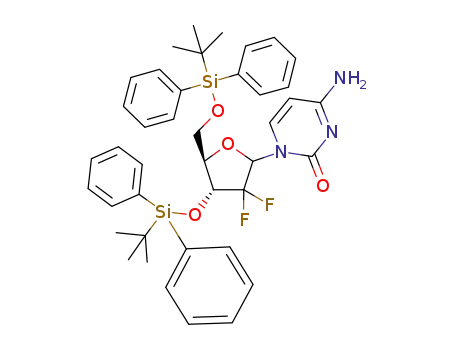
1-[2'-deoxy-2',2'-difluoro-3',5'-bis(tert-butyldiphenylsilyloxy)-ribofuranosyl]-cytosine
-
896109-84-5
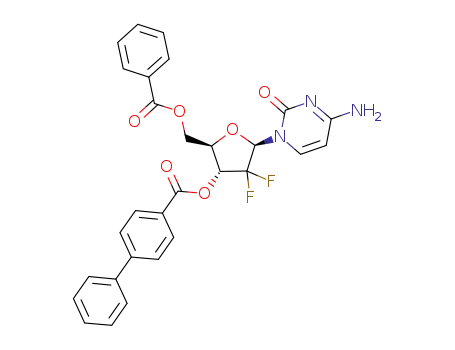
1-(2'-deoxy-2',2'-difluoro-5-benzoyl-3-(4-phenyl)benzoyl-β-D-arabinofuranosyl)-4-aminopyrimidine-2-one
95058-81-4 Downstream products
-
122111-03-9
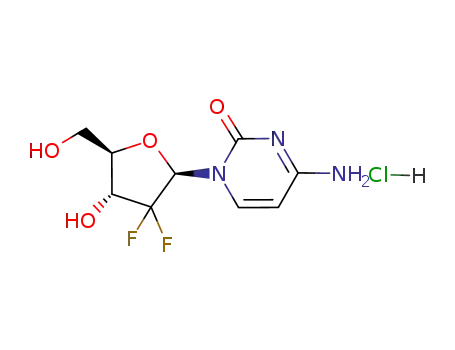
gemcitabine hydrochloride
-
1018907-89-5
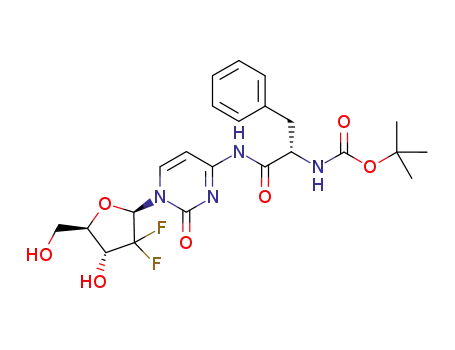
C23H28F2N4O7
-
1421929-70-5
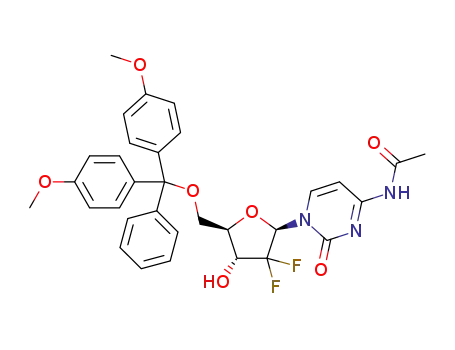
C32H31F2N3O7
-
1421929-71-6
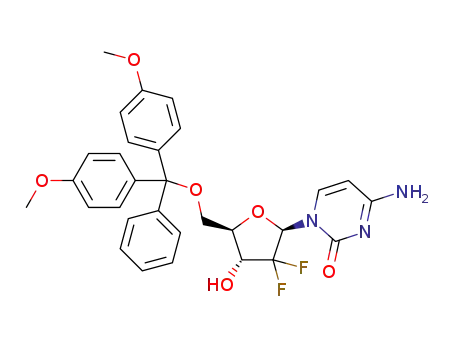
C30H29F2N3O6
Relevant Products
-
High quality Ticagrelor 99%
CAS:274693-27-5
-
Artesunate
CAS:88495-63-0
-
Melatonine
CAS:73-31-4