Product Details
Appearance:white to light yellow crystal powder
Quinine Wholesaler, 130-95-0 in stock, Good Supplier In China
- Molecular Formula:C20H24N2O2
- Molecular Weight:324.423
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:176-177 °C
- Refractive Index:1.6250 (estimate)
- Boiling Point:495.9 °C at 760 mmHg
- PKA:8.52(at 25℃)
- Flash Point:253.7 °C
- PSA:45.59000
- Density:1.21 g/cm3
- LogP:3.11110
130-95-0 Usage
Quinine is one of several alkaloids derived from the bark of the cinchona tree. The cinchona bark was first used against fever in Peru, probably around 1630, but the compound may have been used much earlier by the native Indians. It use as an antimalarial agent spans several hundred years, but it has been replaced in recent years by other substances such as chloroquine. Because some Plasmodium strains have developed resistance to several malaria medications, quinine use is being revived. About 60% of quinine production is used for medicinal purposes, and the drug is available by prescription. In addition to its use as an antimalarial agent, quinine medications are used to treat leg cramps, muscle cramps associated with kidney failure, hemorrhoids, heart palpitations, and as an analgesic.
Definition
ChEBI: A cinchona alkaloid that is cinchonidine in which the hydrogen at the 6-position of the quinoline ring is substituted by methoxy.
InChI:InChI=1/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/p+1/t13-,14-,19-,20+/m0/s1
130-95-0 Relevant articles
pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum
ABS Sidhu,SG Valderramos,DA Fidock
Molecular Microbiology 2010/5/6
Results with pfmdr1 -recombinant clones indicate a significant role for the N1042D mutation in contributing to resistance to quinine and its diastereomer quinidine.
C?H Activation Enables a Concise Total Synthesis of Quinine and Analogues with Enhanced Antimalarial Activity
O' Donovan, Daniel H.,Aillard, Paul,Berger, Martin,de la Torre, Aurélien,Petkova, Desislava,Knittl-Frank, Christian,Geerdink, Danny,Kaiser, Marcel,Maulide, Nuno
, p. 10737 - 10741 (2018)
We report a novel approach to the classical natural product quinine that is based on two stereoselective key steps, namely a C?H activation and an aldol reaction, to unite the two heterocyclic moieties of the target molecule.
METHOD FOR SCREENING SALTY-TASTE MODIFYING SUBSTANCE
-
, (2018/04/19)
A method for screening an objective substance such as a salty-taste modifying substance is provided. It is identified by using a TMC6 protein whether a test substance is an objective substance such as a salty-taste modifying substance.
Cellulose type chiral stationary phase based on reduced graphene oxide@silica gel for the enantiomer separation of chiral compounds
Li, Yuanyuan,Li, Qiang,Zhu, Nan,Gao, Zhuxian,Ma, Yulong
, p. 996 - 1004 (2018/07/29)
The graphene oxide (GO) was covalently coupled to the surfaces of silica gel (SiO2) microspheres by amide bond to get the graphene oxide@silica gel (GO@SiO2). Therefore, the obtained CSP shows special selectivity for benzene-enriched enantiomers, improves separation selectivity and efficiency, and rGO plays a synergistic effect with cellulose derivatives on enantioseparation.
130-95-0 Process route
-
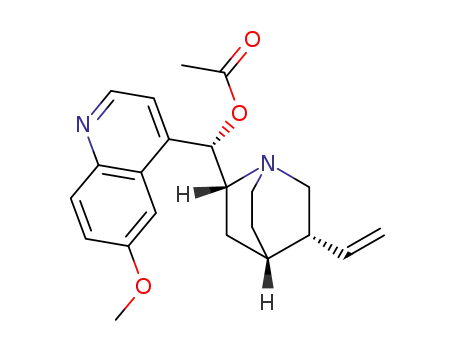
- 56652-53-0
quinidine acetate

-
- 56-54-2,130-95-0,572-59-8,572-60-1,42151-59-7,47342-58-5,72402-50-7,72402-51-8,72402-52-9,72402-53-0,101143-86-6,101143-87-7,101143-88-8,146925-10-2
quinindine
| Conditions | Yield |
|---|---|
|
With methanol; potassium carbonate; at 20 ℃; for 1.5h; Inert atmosphere;
|
95% |
-
![6-Methoxy-4-[(2S,3S)-3-((3R,4S)-3-vinyl-piperidin-4-ylmethyl)-oxiranyl]-quinoline](/upload/2023/1/b7d52d9a-de3f-4e09-9036-99794ade512e.png)
-
6-Methoxy-4-[(2S,3S)-3-((3R,4S)-3-vinyl-piperidin-4-ylmethyl)-oxiranyl]-quinoline

-

- 56-54-2,130-95-0,572-59-8,572-60-1,42151-59-7,47342-58-5,72402-50-7,72402-51-8,72402-52-9,72402-53-0,101143-86-6,101143-87-7,101143-88-8,146925-10-2
quinindine
| Conditions | Yield |
|---|---|
|
In acetonitrile; at 185 ℃; for 0.333333h; Microwave irradiation;
|
|
|
With N,N-dimethyl-formamide; at 160 ℃;
|
130-95-0 Upstream products
-
56-54-2
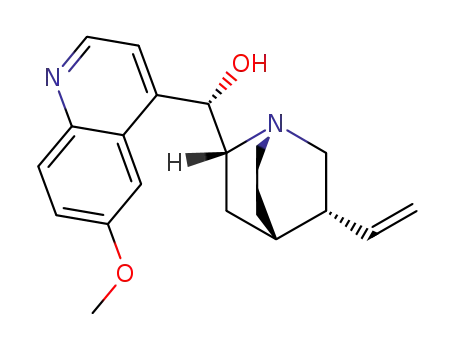
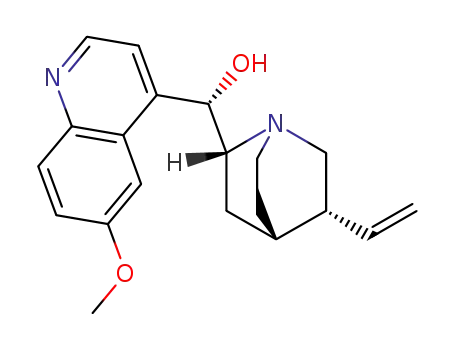
quinidine
-
112661-57-1
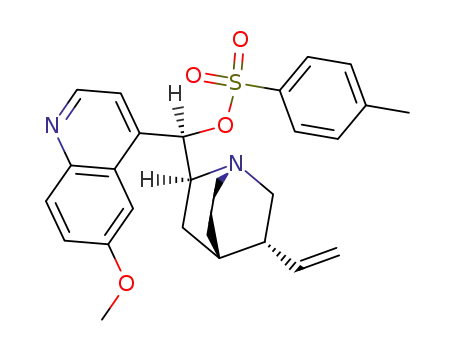
O-tosylquinine
-
7732-18-5
water
-
71-41-0
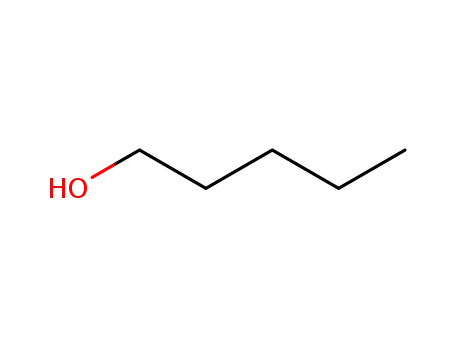
pentan-1-ol
130-95-0 Downstream products
-
78034-56-7
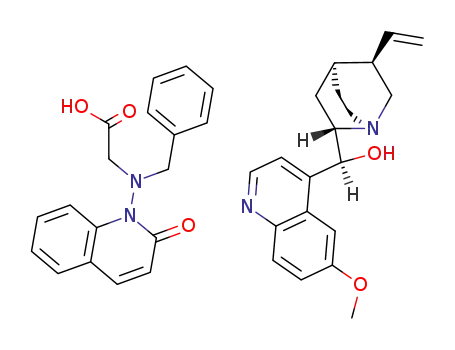
(S)-(6-Methoxy-quinolin-4-yl)-((1S,2S,4S,5R)-5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl)-methanol; compound with [benzyl-(2-oxo-2H-quinolin-1-yl)-amino]-acetic acid
-
84-31-1
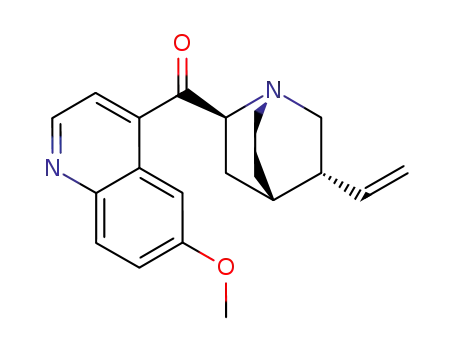
6'-methoxycinchonidone
-
14528-53-1
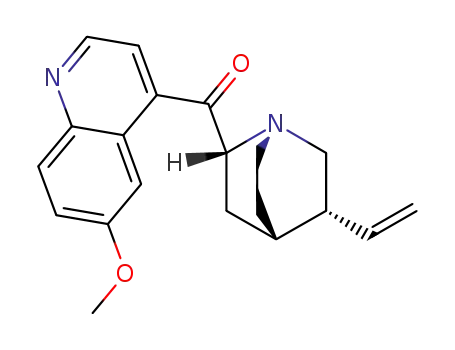
6'-methoxycinchoninone
-
84-55-9
viquidil
Relevant Products
-
Cyclopropane, isocyanato- 4747-72-2
CAS:4747-72-2
-
Tetracaine
CAS:94-24-6
-
Metronidazole
CAS:443-48-1