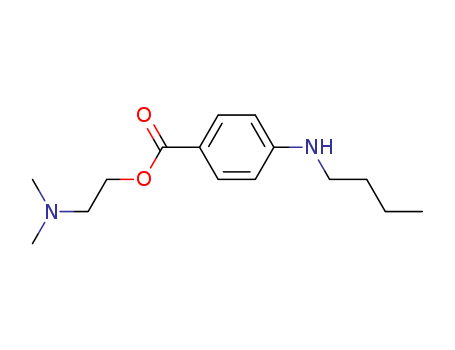
Product Details
Appearance:White crystalline powder
Factory sells Tetracaine 94-24-6 low price, Wholesaler
- Molecular Formula:C15H24N2O2
- Molecular Weight:264.368
- Appearance/Colour:White crystalline powder
- Vapor Pressure:2.87E-06mmHg at 25°C
- Melting Point:41.0 to 45.0 °C
- Refractive Index:1.537
- Boiling Point:389.4 °C at 760 mmHg
- PKA:pKa 8.33±0.03(H2O t = 20.0 I = 0.10 (KCl)) (Uncertain)
- Flash Point:189.3 °C
- PSA:41.57000
- Density:1.044 g/cm3
- LogP:2.69000
Tetracaine Usage
Tetracaine is a local anesthetic or numbing medicine that is used to numb the throat, eyes, or nose. Normally, the drug is administered before starting a surgery to decrease pain from the procedure. It is used to numb various body parts before a surgical procedure or a medical test. Conversely, tetracaine ophthalmic drops are used as local anesthetic for the eyes.
Mechanism of Action
Tetracaine acts by blocking the nerve systems. It does this by blocking the sodium ion channels needed for the conduction and initiation of neural impulses, thereby affecting the local anesthesia. Tetracaine acts by altering the function of calcium release channels that controls the release of calcium frim intracellular cells.
Precautions
No studies have revealed whether the medicine can affect an unborn child; however, it is important to inform the doctor if pregnant while using it. Informing the doctor is paramount if breastfeeding a baby, as no known research has illustrated whether tetracaine can pass into breast milk or harm a nursing baby. To make tetracaine safe, it is important to inform the doctor if one has a history of spinal or brain injury, heart diseases, eye problems, a blood vessel disorder, open sores, skin injury in the area where the medicine will be applied. Tetracaine should never be orally taken since it is typically designed for use on the skin only. If the medicine gets in the mouth, nose, vagina, or rectum, rinse with water.
Side Effects
Seek emergency medical advice in case of any of the following allergic reactions: difficult breathing, hives, swelling of the throat, tongue, lips, or face. Stop using the drug and call the physician if one has severe burning, swelling, stinging, or other irritation of the treated skin. Other alarming symptoms include eye watering, irritation, and increased sensitivity to light. Do not use the medicine in smaller or larger amounts or for longer than recommended.
Definition
ChEBI: A benzoate ester in which 4-N-butylbenzoic acid and 2-(dimethylamino)ethanol have combined to form the ester bond; a local ester anaesthetic (ester caine) used for surface and spinal anaesthesia.
Biochem/physiol Actions
Tetracaine also refers as 4-(Butylamino)benzoic acid 2-(dimethylamino)ethyl ester interfere with calcium movement in muscle and non-muscle cells and can inhibit potassium-induced as well as caffeine-induced shortening of outer hair cells (OHC′s). But, the product can′t inhibit electrically-induced shortening of OHC′s.The product can also stimulate caspase activation and inhibits pro-survival signalling pathways which in turn induce human renal cell apoptosis.
Veterinary Drugs and Treatments
Tetracaine is more irritating than proparacaine but is sometimes used in veterinary medicine. It is indicated to produce local anesthesia of short duration for ophthalmic procedures including measurement of intraocular pressure (tonometry), removal of foreign bodies and sutures, and conjunctival and corneal scraping in diagnosis and gonioscopy. Tetracaine is also indicated to produce local anesthesia prior to surgical procedures in humans such as cataract extraction and pterygium excision, usually as an adjunct to locally injected anesthetics. Ophthalmic solutions used for intraocular procedures should be preservative-free. Preservatives may cause damage to the corneal epithelium if a significant quantity of solution enters the eye through the incision.
Dosage
Always follow all direction on the prescription label. Do not use the medicine in smaller or larger amounts or for longer than recommended.
InChI:InChI=1/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H3
94-24-6 Relevant articles
A Comparison of the Neurotoxic Effects on the Spinal Cord of Tetracaine, Lidocaine, Bupivacaine, and Ropivacaine Administered Intrathecally in Rabbits
Yamashita, Atsuo,Matsumoto, Mishiya,Matsumoto, Satoshi,Itoh, Makoto,Kawai, Koji,Sakabe, Takefumi
, Anesthesia & Analgesia August 2003
In this study, we compared the effects of local anesthetics on glutamate concentrations in CSF microdialysate and neurologic and histopathologic outcome. Rabbits were assigned into 5 groups (n = 6 in each) and intrathecally received 0.3 mL of NaCl solution (control), 2% tetracaine, 10% lidocaine, 2% bupivacaine, or 2% ropivacaine. Neurologic and histopathologic assessments were performed 1 wk after the administration.
Visible Light-Mediated (Hetero)aryl Amination Using Ni(II) Salts and Photoredox Catalysis in Flow: A Synthesis of Tetracaine
Park, Boyoung Y.,Pirnot, Michael T.,Buchwald, Stephen L.
, p. 3234 - 3244 (2020/02/04)
We report a visible light-mediated flow process for C-N cross-coupling of (hetero)aryl halides with a variety of amine coupling partners through the use of a photoredox/nickel dual catalyst system. Scale up of the reaction, demonstrated through the synthesis of tetracaine, is easily achieved, delivering the C-N cross-coupled products in consistently high yield of 84% on up to a 10 mmol scale.
Preparation method of tetracaine hydrochloride
-
, (2017/04/22)
The invention relates to the technical field of preparation method of tetracaine hydrochloride. The preparation method comprises the preparation steps: carrying out a reaction of p-nitrobenzoyl chloride (2) and 2-dimethylamino-1-ethanol (3) to generate p-nitrobenzoic acid-2-dimethylamino ethyl (4), reducing the compound (3) to obtain p-aminobenzoic acid-2-dimethylamino ethyl (4), generating pontocaine (7) from a compound (5) and 1-bromobutane (6) under alkaline conditions, and finally carrying out a reaction of the pontocaine (7) with HCl to generate tetracaine hydrochloride (1).
Block of cyclic nucleotide-gated channels by tetracaine derivatives: Role of apolar interactions at two distinct locations
Strassmaier, Timothy,Kirk, Sarah R.,Banerji, Tapasree,Karpen, Jeffrey W.
, p. 645 - 649 (2008/09/19)
A series of new tetracaine derivatives was synthesized to explore the effects of hydrophobic character on blockade of cyclic nucleotide-gated (CNG) channels. Shape also plays an important role. While gradual increases in length of the butyl tail lead to increased potency, substitution of the butyl tail with branched alkyl or cyclic groups is deleterious.
94-24-6 Process route
-
- 109-65-9
1-bromo-butane

-
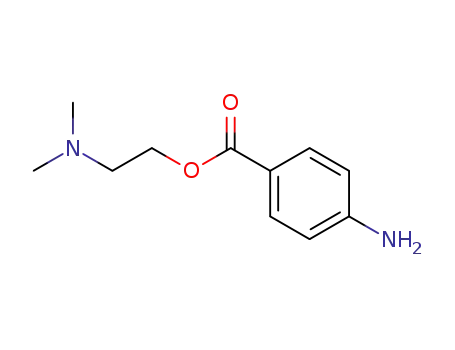
- 10012-47-2
Dimethylprocaine

-
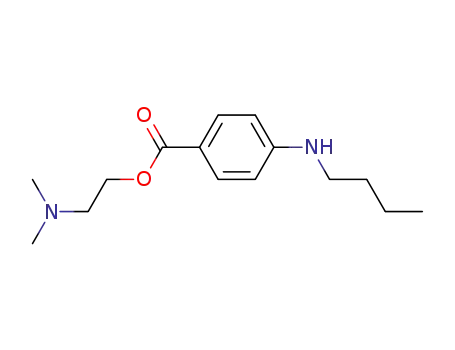
- 94-24-6
amethocaine
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 50 ℃; for 5h;
|
82.8% |
|
With butan-1-ol;
|
-
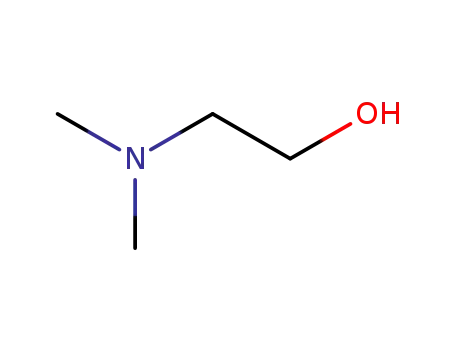
- 108-01-0
2-(N,N-dimethylamino)ethanol

-
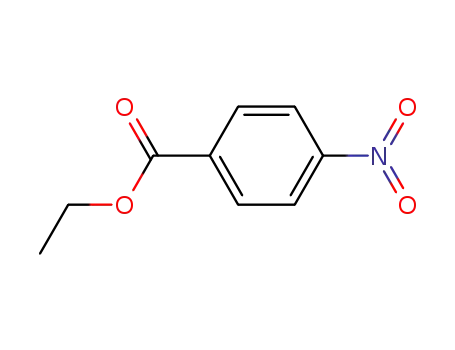
- 99-77-4
ethyl 4-nitrobenzoate

-
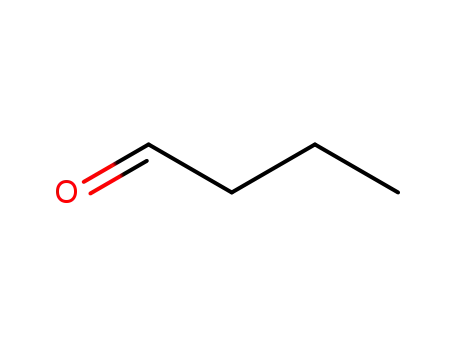
- 123-72-8
butyraldehyde

-

- 94-24-6
amethocaine
| Conditions | Yield |
|---|---|
|
With hydrogen; palladium-containing anion exchanger AV-17-8; In ethanol; at 45 ℃; under 760 Torr; Further Variations:; Catalysts; concentrations; Kinetics;
|
94% |
|
With hydrogen; palladium-containing anion exchanger AV-17-8; In ethanol; at 45 ℃; under 760 Torr;
|
94% |
94-24-6 Upstream products
-
108-01-0

2-(N,N-dimethylamino)ethanol
-
4740-24-3
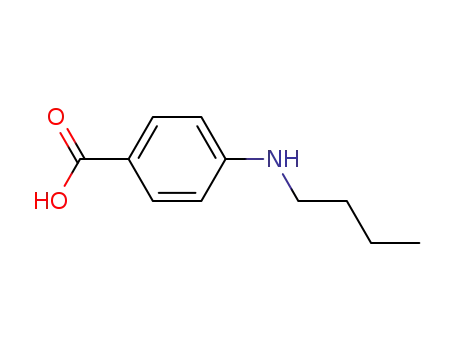
4-(N-butylamino)benzoic acid
-
38152-22-6
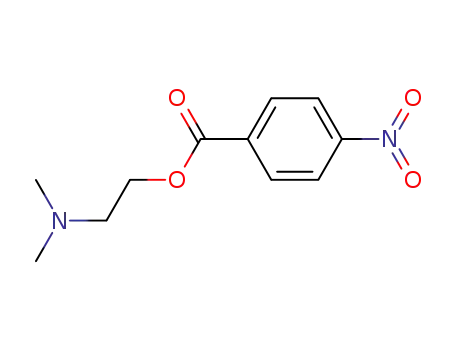
4-nitrobenzoic acid 2-(dimethylamino)ethyl ester
-
122-04-3
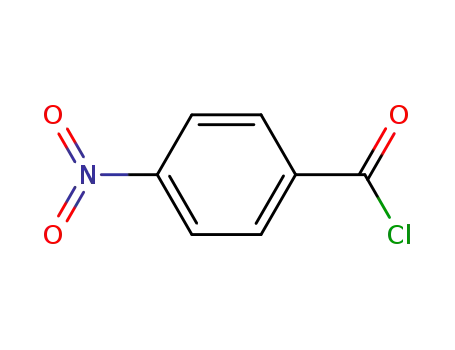
4-nitro-benzoyl chloride
94-24-6 Downstream products
-
55750-02-2
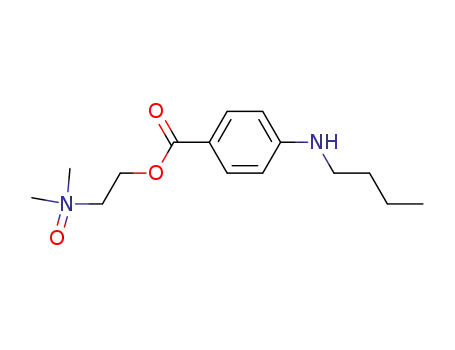
4-butylamino-benzoic acid-[2-(dimethyl-oxy-amino)-ethyl ester]
-
136-47-0
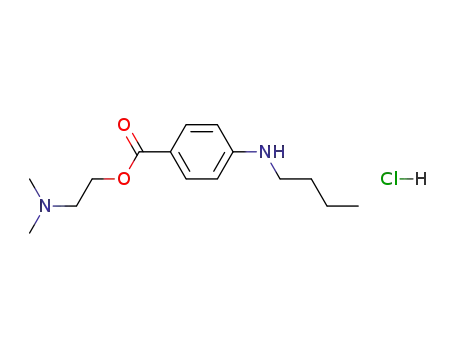
tetracaine hydrochloride
Relevant Products
-
Cyclopropane, isocyanato- 4747-72-2
CAS:4747-72-2
-
Tetrandrine /D-Tetrandrine
CAS:518-34-3
-
Quinine
CAS:130-95-0