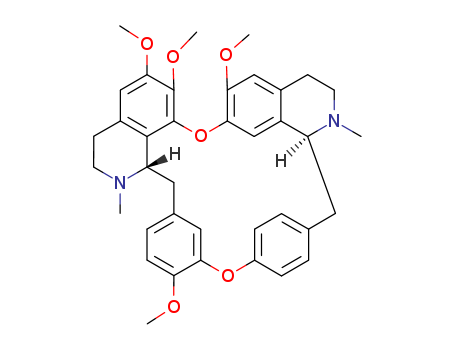
518-34-3
- Product Name:Tetrandrine /D-Tetrandrine
- Molecular Formula:C38H42N2O6
- Purity:99%
- Molecular Weight:
Product Details
Appearance:solid
Tetrandrine in bulk supply with low price, 518-34-3 D-Tetrandrine On Sale
- Molecular Formula:C38H42N2O6
- Molecular Weight:622.761
- Appearance/Colour:solid
- Vapor Pressure:4.88E-20mmHg at 25°C
- Melting Point:219-222 °C
- Refractive Index:1.585
- Boiling Point:710.5 °C at 760 mmHg
- PKA:7.70±0.20(Predicted)
- Flash Point:175.8 °C
- PSA:61.86000
- Density:1.172 g/cm3
- LogP:7.03820
Tetrandrine /D-Tetrandrine 518-34-3 Usage
Tetrandrine, isolated from the root of Stephania tetrandra S Moore, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. Recently it has been discovered that pronounced drug-dependence and related toxic effects occur in both dogs and rhesus monkeys with this alkaloid on intravenous injection with a dose of 10-150 mg/kg. Tetrandrine is used for the treatment of hypertension, angina, termination of paroxysmal supraventricular tachycardia, pulmonary fibrosis, and other diseases in clinical application, and it also has strong antitumor effects. Tetrandrine was also approved for lowering blood glucose and free radical damage; its treatment effect on silicosis is significant and superior to conventional immunosuppressive and cytotoxic drugs. Tetrandrine is used in China to treat high blood pressure. Its other pharmacological effects are to be explored in further study.
Indications
Tetrandrine has analgesic, anti-inflammatory, and anti-allergic effects and has a wide range of usage on the cardiovascular system owing to its antihypertensive, anti-myocardial ischemia/reperfusion injury and antiarrhythmic effects. Tetrandrine is a calcium channel protein inhibitor. Tetrandrine blocks the L-type (IC50= 0.3-8 µM) and T-type (IC50= 2.5-20 µM) calcium channels. This product is included in national standards for chemical drugs (Volume 14), British Pharmacopoeia (2017), and European Pharmacopoeia (9.0th ed.).
InChI:InChI=1/C38H42N2O6/c1-39-15-13-25-20-32(42-4)34-22-28(25)29(39)17-23-7-10-27(11-8-23)45-33-19-24(9-12-31(33)41-3)18-30-36-26(14-16-40(30)2)21-35(43-5)37(44-6)38(36)46-34/h7-12,19-22,29-30H,13-18H2,1-6H3/t29?,30-/m0/s1
518-34-3 Relevant articles
Potential role of tetrandrine in cancer therapy
ChenYJ
Acta Pharmacologica Sinica 2002
The beneficial effects of tetrandrine on tumor cell cytotoxicity and radiosensitization,multidrug resistance,normal tissue radioprotection,and angiogenesis are most promising and deserve great attention.Tetrandrine has potential either as a tumoricidal agent or as an adjunct to chemotherapy and radiotherapy.
Clinical and pharmacological studies with D tetrandrine
RC Deconti,F Muggia,FJ Cummings
Proceedings of the American Association for Cancer Research 1975/01/01
Using animal toxicity data, 2 dosage schedules with a 1 hr infusion time were chosen. Single dose and 5 day schedules were gradually escalated from 50-875 mg/M in 32 patients.
A CHEMISTRY OF BISBENZYLISOQUINOLINE ALKALOID: ISOMERIZATION OF BERBAMINE TO PENDULINE IN METHANOL BY RADICAL REACTION
Kashiwaba, Noriaki,Morooka, Shigeo,Kimura, Michiko,Ono, Minoru,Murakoshi, Yoshie,et al.
, p. 2043 - 2048 (2007/10/03)
Berbamine (1), a bisbenzylisoquinoline alkaloid, when treated with methanol, was isomerized to penduline (2), the C-1 epimer of 1.
518-34-3 Process route
-

- 186581-53-3,908094-01-9,334-88-3
diazomethane

-
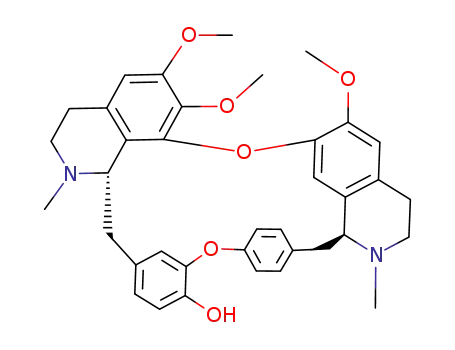
- 26137-45-1
(+)-penduline

-
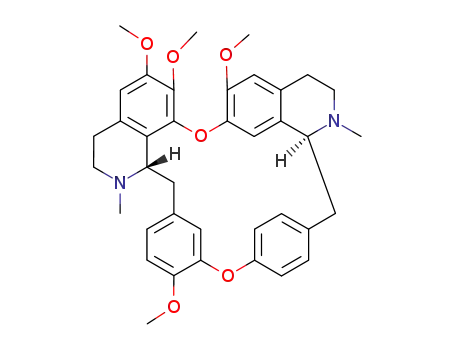
- 518-34-3,5990-67-0
Tetrandrin
| Conditions | Yield |
|---|---|
|
With diethyl ether; In methanol; for 24h;
|
100% |

-

- 518-34-3,5990-67-0
Tetrandrin
| Conditions | Yield |
|---|---|
|
|
518-34-3 Upstream products
-
186581-53-3

diazomethane
-
26137-45-1

(+)-penduline
-
67-56-1
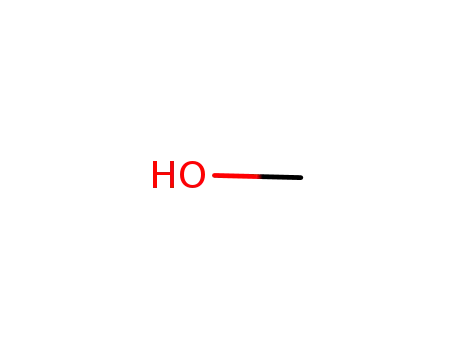
methanol
-
50-00-0
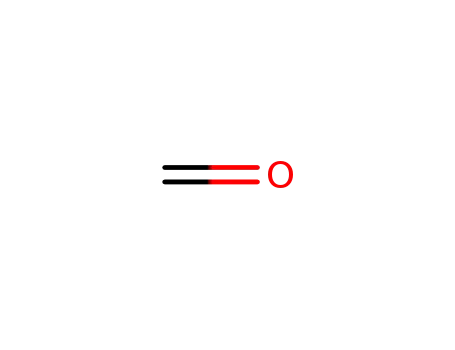
formaldehyd
518-34-3 Downstream products
-
477-57-6
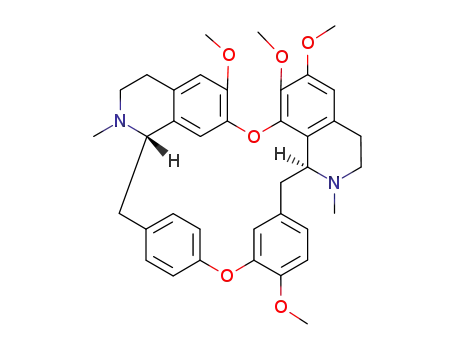
tetrandrine
-
3423-07-2
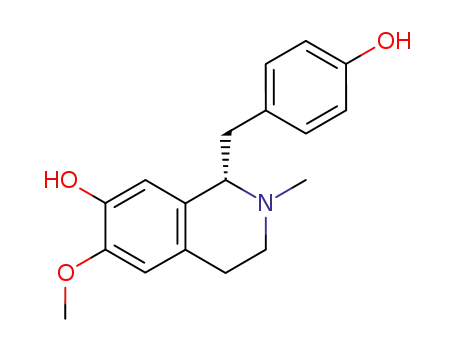
(S)-N-methylcoclaurine
-
3423-02-7
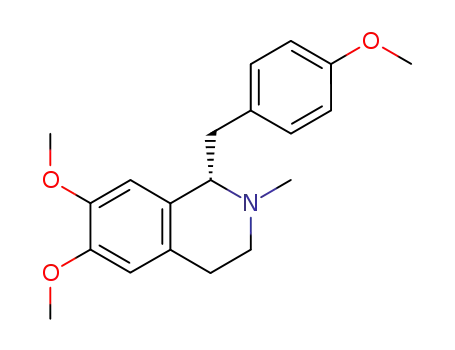
(R)-(-)-O-methylarmepavine
-
436-77-1
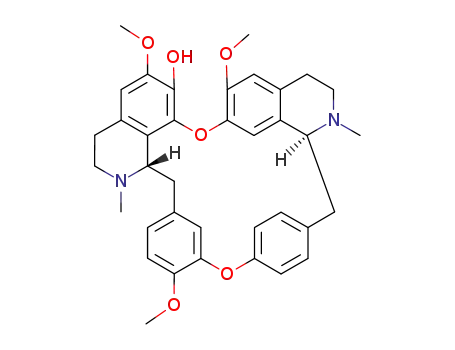
fangchinoline
Relevant Products
-
Cyclopropane, isocyanato- 4747-72-2
CAS:4747-72-2
-
Orlistat
CAS:96829-58-2
-
Tetracaine
CAS:94-24-6