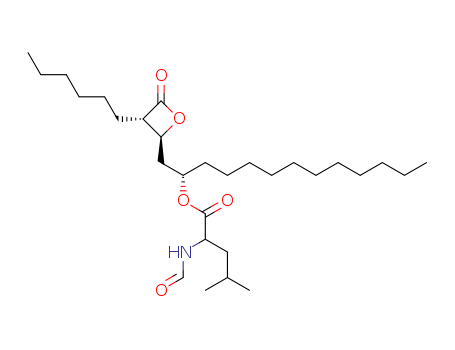
Product Details
Appearance:off-white solid
Orlistat Hot Sale, 96829-58-2 in stock On Sale
- Molecular Formula:C29H53NO5
- Molecular Weight:495.744
- Appearance/Colour:off-white solid
- Vapor Pressure:4.23E-15mmHg at 25°C
- Melting Point:<50 °C
- Refractive Index:1.469
- Boiling Point:615.9 °C at 760 mmHg
- PKA:14.59±0.23(Predicted)
- Flash Point:326.3 °C
- PSA:81.70000
- Density:0.976 g/cm3
- LogP:7.90870
Orlistat 96829-58-2 Usage
Orlistat is a type of lipase inhibiting weight loss drug and is a hydrated derivative of lipostatin. Its commercial name is Sainike and first went on sale in New Zealand in 1998. Orlistat is a digestive lipase inhibitor. It inhibits diacylglycerol lipase α (DAGLα), DAGLβ, α/β-hydrolase domain-containing protein 12 (ABHD12), ABHD16A, and platelet-activating factor acetylhydrolase (PAF-AH; IC50s = 0.06, 0.1, 0.08, 0.03, and 0.05 μM, respectively), as well as pancreatic lipase and hormone-sensitive lipase (IC50s = 0.65 and 2.1 μg/ml, respectively) but does not inhibit fatty acid amide hydrolase (FAAH) or KIAA1363 (IC50s = >100 μM for both).
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.Orlistat is a specific lipase inhibitor derived from lipostatin, which is naturally produced by Streptomyces toxytricini. Orlistat has a long-term weight-control effect that reduces and maintains weight and prevents against rebounding. Using Orlistat may reduce absorption of vitamin A, D and E; take supplements when using this drug. Any preparations containing vitamin A, D or E (such as compound vitamin preparations), should be taken 2 hours after this drug or before bed. Type-2 diabetes patients may need to decrease dosage of antidiabetic drugs (such as sulfonylurea). Combined use with cyclosporine may lower the blood concentration of the latter drug. Combined use with amiodarone may reduce absorption of the latter drug, thus decreasing its curative effects.New study suggests that a lack of information about orlistat's side effects may be one of the reasons why some people actually gain weight after being prescribed the medication to help them fight obesity.
InChI:InChI=1/C29H53NO5/c1-5-7-9-11-12-13-14-15-16-18-24(34-29(33)26(30-22-31)20-23(3)4)21-27-25(28(32)35-27)19-17-10-8-6-2/h22-27H,5-21H2,1-4H3,(H,30,31)/t24-,25?,26-,27-/m0/s1
96829-58-2 Relevant articles
Total Synthesis of Tetrahydrolipstatin, Its Derivatives, and Evaluation of Their Ability to Potentiate Multiple Antibiotic Classes against Mycobacterium Species
Khan, Saniya S.,Landgraf, Alexander D.,Ronning, Donald R.,Sucheck, Steven J.,Sudasinghe, Thanuja D.
, (2021/09/27)
Tetrahydrolipstatin (THL, 1a) has been shown to inhibit both mammalian and bacterial α/β hydrolases. Compound 1e applied at concentrations 4-fold lower than its MIC enhanced the MIC of the synergistic antibiotic by 2-256-fold. In addition to observing synergy with first-line drugs, rifamycin and isoniazid, the MIC of vancomycin against M. tuberculosis H37Ra was 65 μg/mL; however, the MIC was lowered to 0.25 μg/mL in the presence of 2.1 μg/mL 1e demonstrating the potential of targeting mycobacterial hydrolases involved in mycomembrane and peptidoglycan biosynthesis.
Orlistat and Acute Kidney Injury: An Analysis of 953 Patients
Weir,A Matthew
Archives of Internal Medicine 2011
Arch Intern Med. 2011 Apr 11;171(7):703-4. doi: 10.1001/archinternmed.2011.103. Letter; Research Support, Non-U.S. Gov't
Method for preparing weight-reducing medicine orlistat
-
Paragraph 0009; 0020; 0021; 0022; 0223; 0024; 0025-0037, (2017/08/28)
The invention discloses a method for preparing a weight-reducing medicine orlistat. The method for preparing the orlistat, which is provided by the invention, is mild in reaction condition and simple to operate; the reaction yield is effectively improved; the hydrazine hydrate is adopted as a reducing agent and a hydrogen source; reaction by-products are few; the after treatment is simple.
96829-58-2 Process route
-
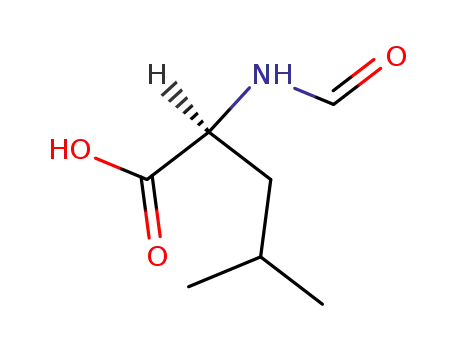
- 5338-45-4,44978-39-4,89921-44-8,6113-61-7
N-formyl-L-leucine

-
![(3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-2-oxetanone](/upload/2023/1/8ae6c530-7333-492a-a563-03ec97c78563.png)
- 68711-40-0,104871-99-0,104872-00-6,104872-03-9,104872-05-1,104872-07-3,104872-19-7,104872-06-2
(3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-2-oxetanone

-
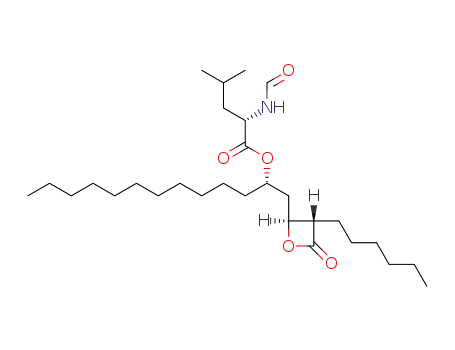
- 104872-04-0,104872-27-7,104872-28-8,111466-61-6,111466-62-7,111466-63-8,130193-40-7,130193-41-8,130193-42-9,130193-43-0,96829-58-2,1225451-00-2
Orlistat
| Conditions | Yield |
|---|---|
|
With di-isopropyl azodicarboxylate; triphenylphosphine; In tetrahydrofuran; at 0 - 20 ℃; Inert atmosphere;
|
94% |
|
With di-isopropyl azodicarboxylate; triphenylphosphine; In tetrahydrofuran; at 20 ℃; for 3h;
|
93% |
|
With di-isopropyl azodicarboxylate; thiamine diphosphate; In tetrahydrofuran; at 0 - 20 ℃; for 3h;
|
90% |
|
With di-isopropyl azodicarboxylate; triphenylphosphine; In tetrahydrofuran; at 23 ℃;
|
90% |
|
With di-isopropyl azodicarboxylate; triphenylphosphine; In tetrahydrofuran; at 20 ℃;
|
90% |
|
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; for 18h; Ambient temperature;
|
89% |
|
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; Ambient temperature;
|
85.8% |
|
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; at 0 ℃; for 4h;
|
84% |
|
With di-isopropyl azodicarboxylate; triphenylphosphine; In tetrahydrofuran; at 0 - 20 ℃;
|
84% |
|
With di-isopropyl azodicarboxylate; triphenylphosphine; In tetrahydrofuran; at 0 ℃; for 6h;
|
83% |
|
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; for 2h; Ambient temperature;
|
80% |
|
N-formyl-L-leucine; (3S,4S)-3-hexyl-4-[(R)-2-hydroxytridecyl]-2-oxetanone; With triphenylphosphine; In xylene; for 2h;
With di-isopropyl azodicarboxylate; In tetrahydrofuran; at 0 - 20 ℃; for 12.1667h;
|
80% |
|
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; 1.) 0 deg C, 1 h; 2.) room temp. 2 h;
|
77% |
|
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; at 0 - 20 ℃;
|
73% |
|
With thiamine diphosphate; diethylazodicarboxylate; at 0 - 20 ℃;
|
65% |
|
With di-isopropyl azodicarboxylate; triphenylphosphine; In tetrahydrofuran; toluene; at 0 - 20 ℃; for 3h; Inert atmosphere;
|
17.3 mg |
|
With triphenylphosphine; diethylazodicarboxylate; enantioselective reaction;
|
|
|
Mitsunobu esterification;
|
-
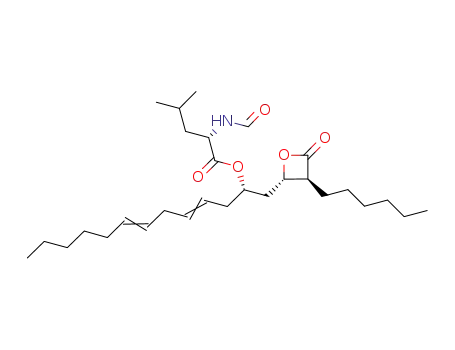
- 1243011-53-1
lipstatin

-

- 104872-04-0,104872-27-7,104872-28-8,111466-61-6,111466-62-7,111466-63-8,130193-40-7,130193-41-8,130193-42-9,130193-43-0,96829-58-2,1225451-00-2
Orlistat
| Conditions | Yield |
|---|---|
|
With hydrogen; 5%-palladium/activated carbon; In ethanol; at 20 ℃; for 6h;
|
83% |
96829-58-2 Upstream products
-
64-18-6
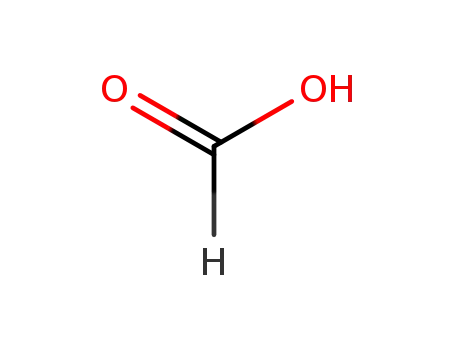
formic acid
-
2018-66-8
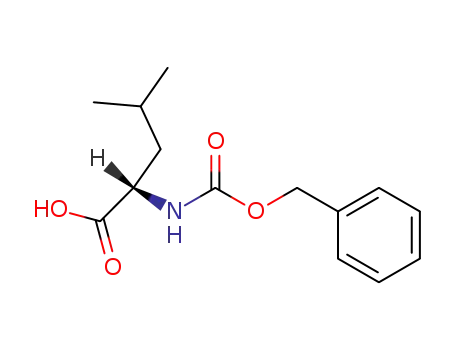
Z-Leu-OH
-
104871-99-0
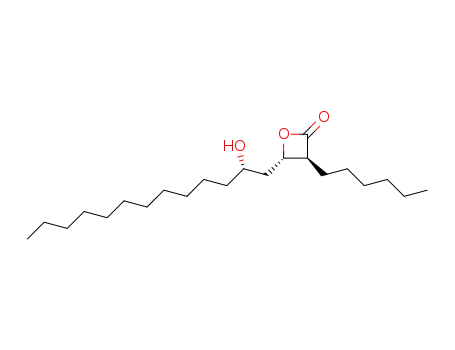
(3S,4S)-3-hexyl-4-[(2S)-2-hydroxytridecyl]oxetan-2-one
-
5338-45-4

N-formyl-L-leucine
96829-58-2 Downstream products
-
68711-41-1
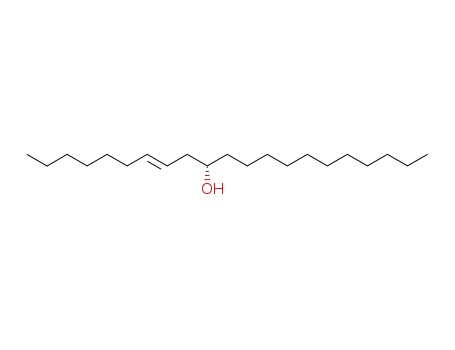
(S,E)-Henicos-7-en-10-ol
-
130676-63-0
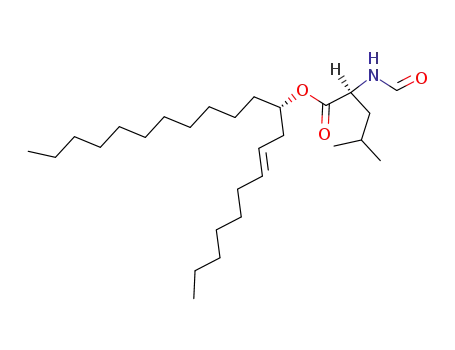
N-Formyl-L-leucine (S,E)-1-(Non-2-enyl)dodecyl Ester
-
130676-64-1
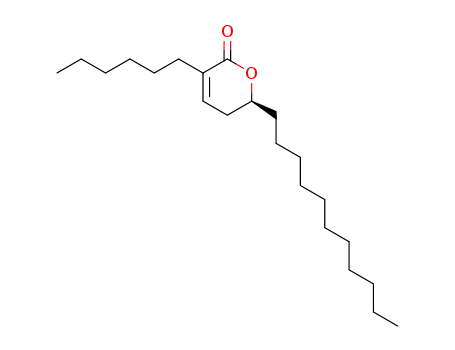
(S)-3-Hexyl-5,6-dihydro-6-undecyl-2H-pyran-2-one
-
130793-26-9
(3S,4R,6S)-3-Hexyl-3,4,5,6-tetrahydro-4-hydroxy-6-undecyl-2H-pyran-2-one
Relevant Products
-
Cyclopropane, isocyanato- 4747-72-2
CAS:4747-72-2
-
Cetilistat
CAS:282526-98-1
-
Tetrandrine /D-Tetrandrine
CAS:518-34-3