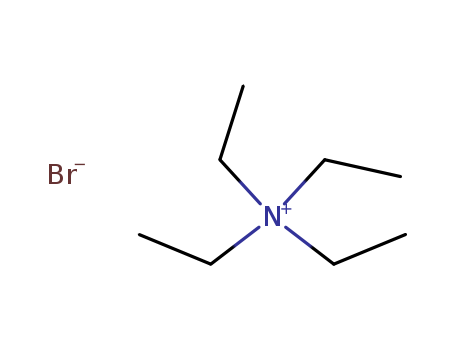
71-91-0
- Product Name:Tetraethylammonium bromide
- Molecular Formula:C8H20BrN
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white to light yellow crystalline solid
Factory sells Tetraethylammonium bromide 71-91-0 in stock, superior manufacturer
- Molecular Formula:C8H20BrN
- Molecular Weight:210.157
- Appearance/Colour:white to light yellow crystalline solid
- Melting Point:285 °C (dec.)(lit.)
- Refractive Index:1,442-1,444
- PSA:0.00000
- Density:1.397 g/cm3
- LogP:-1.11320
71-91-0 Usage
Tetraethylammonium is positively charged due to its central quaternary ammonium. Tetraethylammonium Bromide 71-91-0 , can be used as a source of tetraethylammonium ions for various pharmaceutical studies. It has also the ability to block K+ channels in various tissues. TEAB can also be used as an organic template to synthesize zeolite beta.
InChI:InChI=1/C8H20N.BrH/c1-5-9(6-2,7-3)8-4;/h5-8H2,1-4H3;1H/q+1;/p-1
71-91-0 Relevant articles
Versatile Method for the Simultaneous Synthesis of Two Ionic Liquids, Otherwise Difficult to Obtain, with High Atom Economy
Szpecht, Andrea,Zajac, Adrian,Zielinski, Dawid,Maciejewski, Hieronim,Smiglak, Marcin
, p. 972 - 983 (2019/08/06)
A new synthetic approach and full spectral (NMR, IR, MS) and ion chromatographic characterization (IC) of nitrogen-based ionic liquids bearing allyl- or ethyl- substituent and triflate, tosylate, methyl sulfate or methanesulfonate anion has been presented.This approach ensured high atom economy of the overall process, which makes the proposed methodology sustainable and eco-friendly.
Effect of Tetraethylammonium Bromide on Gastric Secretion and Motility
IR Macdonald,AN Smith
Br Med J 19490917
In this article, we have explored the Effect of Tetraethylammonium Bromide on Gastric Secretion and Motility.
NMR study of the complex formation between tert-butyl hydroperoxide and tetraalkylammonium bromides
Turovskij, Nikolaj A.,Berestneva, Yulia V.,Raksha, Elena V.,Zubritskij, Mikhail Yu.,Grebenyuk, Serhiy A.
, p. 1443 - 1448 (2014/11/27)
The interaction between tert-butyl hydroperoxide and tetraalkylammonium bromides was studied by NMR spectroscopy in acetonitrile-d 3 at 298 K. The complex formation between the hydroperoxide molecule and corresponding quaternary ammonium salt w
71-91-0 Process route
-

- 121-44-8
triethylamine

-
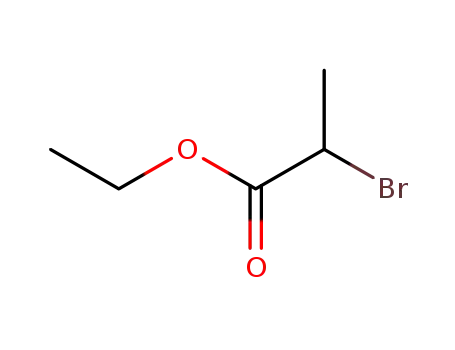
- 535-11-5,41978-69-2
Ethyl 2-bromopropionate

-
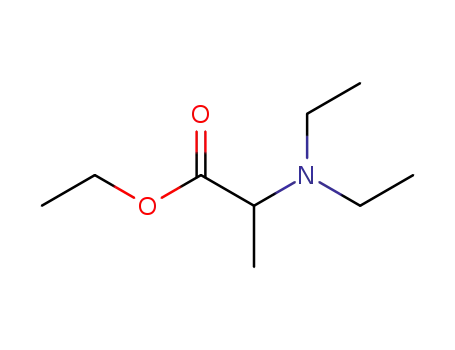
- 82614-48-0
ethyl 2-diethylaminopropanoate

-
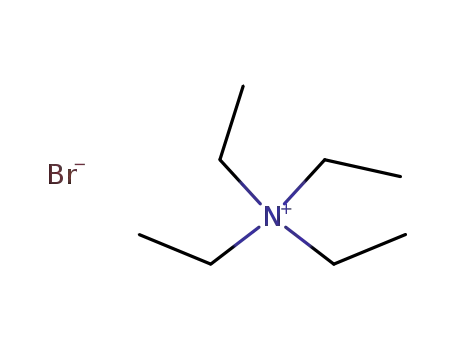
- 71-91-0
tetraethylammonium bromide

-
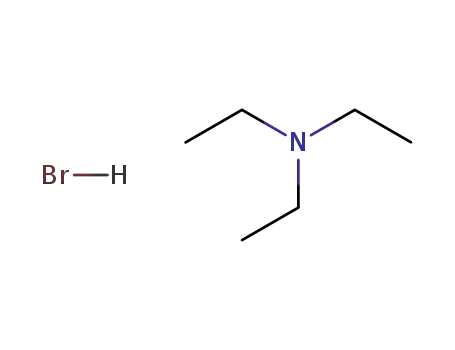
- 636-70-4
triethylamine hydrobromide
| Conditions | Yield |
|---|---|
|
at 107 ℃;
|
-

- 121-44-8
triethylamine

-
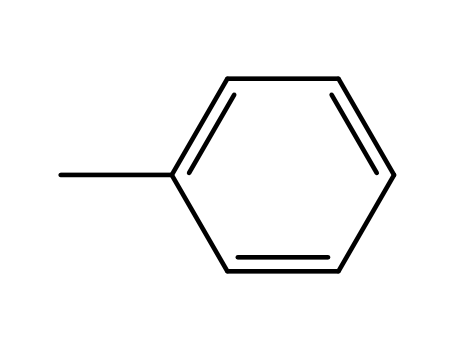
- 108-88-3,15644-74-3,16713-13-6
toluene

-

- 535-11-5,41978-69-2
Ethyl 2-bromopropionate

-

- 82614-48-0
ethyl 2-diethylaminopropanoate

-
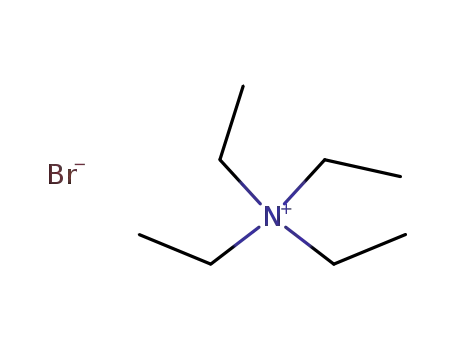
- 71-91-0
tetraethylammonium bromide

-
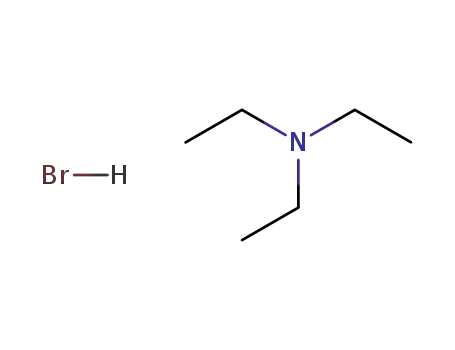
- 636-70-4
triethylamine hydrobromide
| Conditions | Yield |
|---|---|
|
at 50 ℃;
|
71-91-0 Upstream products
-
74-96-4
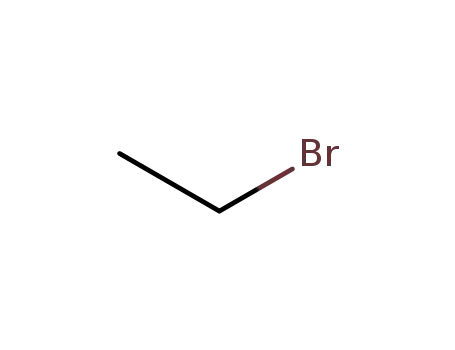
ethyl bromide
-
121-44-8

triethylamine
-
64-67-5
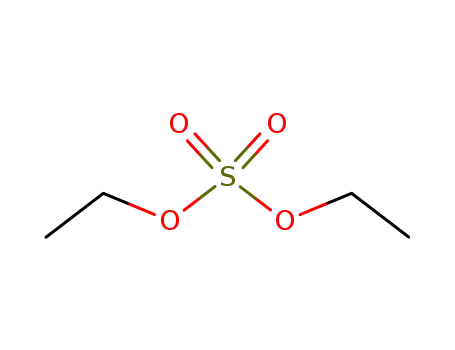
diethyl sulfate
-
540-49-8
cis+trans-dibromoethylene
71-91-0 Downstream products
-
703-56-0
N-phenyl-2-oxazolidinone
-
96-49-1
[1,3]-dioxolan-2-one
-
65368-15-2
C21H18BCl2(1-)*C8H20N(1+)
-
1185-59-7
tetraethylammonium acetate
Relevant Products
-
2-Bromonicotinic acid 35905-85-2
CAS:35905-85-2
-
Tetracaine hydrochloride
CAS:136-47-0
-
Sodium tert-butoxide
CAS:865-48-5