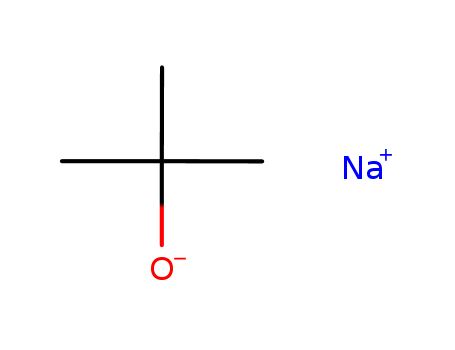
865-48-5
- Product Name:Sodium tert-butoxide
- Molecular Formula:C4H9NaO
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white to light tan crystalline powder
Shanghai Upbio Tech Co.,Ltd (Former Onchem (China)Co.,Ltd) is a comprehensive manufacturer and an international distribution of chemicals throughout the world, The predecessor of Shanghai Upbio Tech Co.,Ltd was Onchem (China) Co.,Ltd in 2010,Specialized in APIs, chemical intermediates, herbal extract and pharmaceutical raw materials.
Hot Sale, Sodium Tert-butylate 865-48-5 for sale, Good Supplier In China
- Molecular Formula:C4H9NaO
- Molecular Weight:96.1046
- Appearance/Colour:white to light tan crystalline powder
- Vapor Pressure:46mmHg at 25°C
- Melting Point:180 °C
- Refractive Index:n20/D1.413
- Boiling Point:84.6 °C at 760 mmHg
- Flash Point:11.7 °C
- PSA:23.06000
- Density:1,104 g/cm3
- LogP:1.21540
Sodium tert-butoxide 865-48-5 Usage Reliable quality 865-48-5 in bulk supply
Sodium tert-butoxide (or sodium t-butoxide) is a strong, non-nucleophilic base. Sodium tert-butoxide can be used as:
A promoter for the synthesis of biaryls by C-H bond arylation of aromatic compounds with haloarenes.
A base in palladium catalyzed amination reactions.
A base in the synthesis of aryl tert-butyl ethers from unactivated aryl halides in presence of palladium catalyst.
In the desulfonylation of N-indoles and N-azaindoles.
As a base in the synthesis of sodium acrylates from olefins and CO2.
Flammability and Explosibility
Highlyflammable
PROCEDURE FOR HANDLING
Avoid all personal contact, including inhalation. Wear protective clothing when risk of overexposure occurs. Use in a well-ventilated area. Avoid smoking, naked lights or ignition sources.
InChI:InChI=1/C4H9O.Na/c1-4(2,3)5;/h1-3H3;/q-1;+1
865-48-5 Relevant articles
Sodium tert-butoxide as stable electrode material in aprotic electrolyte for high cycle stability organic sodium-ion batteries
T Huang, R Zheng, H Chang, D Ma, H Niu
Journal of Power Sources, 2022
We report a new organic active unit sodium tert-butoxide (STB), which can exist stably in aprotic electrolyte to obtain high cycle stability. As the electrode of rechargeable batteries, the STB exhibits a capacity of 206 mAhg−1 at 35 mAg−1, 2.4 V. The battery runs more than 5000 cycles at 210 mAg−1, 135 mAhg−1, no capacity attenuation.
A practical approach for the transamidation of N, N-dimethyl amides with primary amines promoted by sodium tert-butoxide under solvent-free conditions
Rui Zhang , Jun-Chao Zhang , Wei-Yi Zhang , Yu-Qing He , Hua Cheng∗ , Cheng Chen ∗ , Yu-Cheng Gu
Synthesis 2020; 52(21): 3286-3294
A practical sodium tert-butoxide (NaOtBu)-mediated protocol is disclosed for the transamidation of various N,N-dimethyl amides with primary amines to afford the corresponding amides in moderate to good yields at room temperature under solvent-free conditions.
Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide
Jamie H. Docherty, Jingying Peng, Andrew P. Dominey & Stephen P. Thomas
Nature Chemistry volume 9, pages595–600 (2017)
Here, we report a simple method for the use of earth-abundant metal catalysts by general activation with sodium tert-butoxide. Using only robust air- and moisture-stable reagents and pre-catalysts, both known and, significantly, novel catalytic activities have been successfully achieved, covering hydrosilylation, hydroboration, hydrovinylation, hydrogenation and [2π+2π] alkene cycloaddition. This activation method allows for the easy use of earth-abundant metals, including iron, cobalt, nickel and manganese, and represents a generic platform for the discovery and application of non-precious metal catalysis.
865-48-5 Upstream products
-
75-65-0
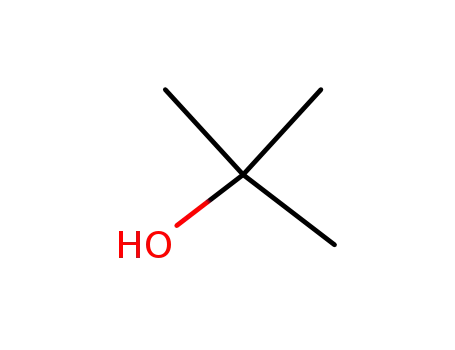
tert-butyl alcohol
-
872-50-4
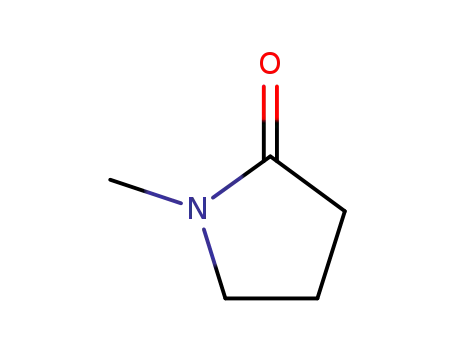
1-methyl-pyrrolidin-2-one
865-48-5 Downstream products
-
59862-83-8
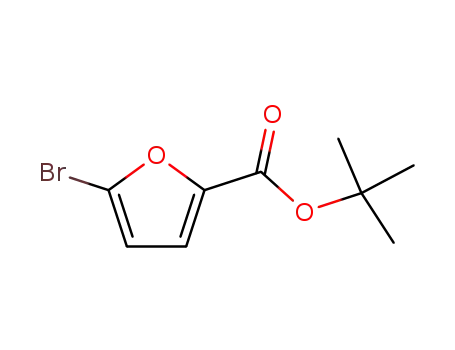
tert-butyl 5-bromofuran-2-carboxylate
-
90612-88-7
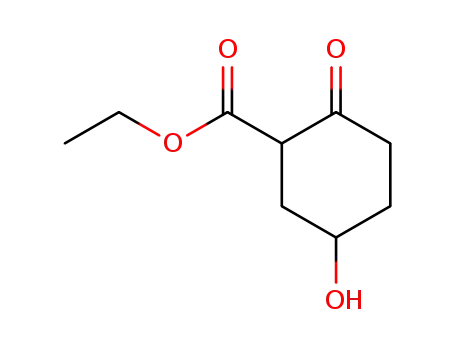
5-hydroxy-2-oxo-cyclohexanecarboxylic acid ethyl ester
-
1694-31-1
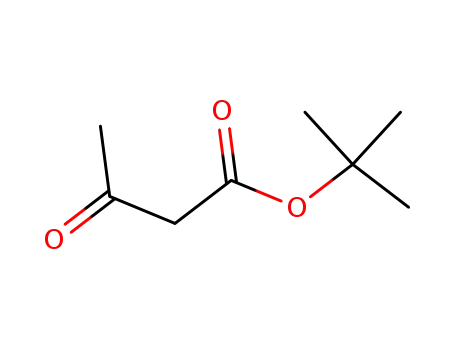
tert-butyl acetoacetate
-
18166-43-3
tris(tert-butoxy)silanol
Relevant Products
-
Tetraethylammonium bromide
CAS:71-91-0
-
Testosterone Enanthate
CAS:315-37-7