136-47-0
- Product Name:Tetracaine hydrochloride
- Molecular Formula:C15H25ClN2O2
- Purity:99%
- Molecular Weight:
Product Details
Appearance:White or off white crystalline powder
Tetracaine hydrochloride 136-47-0 trader, Fast Delivery, low price
- Molecular Formula:C15H25ClN2O2
- Molecular Weight:300.829
- Appearance/Colour:White or off white crystalline powder
- Vapor Pressure:2.87E-06mmHg at 25°C
- Melting Point:149 ºC
- Refractive Index:1.5200 (estimate)
- Boiling Point:389.4 ºC at 760 mmHg
- PKA:8.39(at 25℃)
- Flash Point:189.3 ºC
- PSA:41.57000
- Density:1.1279 (rough estimate)
- LogP:3.49200
Tetracaine hydrochloride 136-47-0 Usage
Tetracaine hydrochloride is also known as ropivacaine hydrochloride, pantocaine, pantocaine and four ropivacaine hydrochloride. Tetracaine hydrochloride is a white, crystalline powder, odourless; hygroscopic; it has a slightly bitter taste and causes local numbness after being placed on the tongue. Soluble in water, soluble in ethanol (~750 g/l), practically insoluble in ether. Tetracaine hydrochloride is a local anesthetic used topically in opthalmology. It works by blocking nerve signals in your body. It is a highly effective local anesthetic that reversibly blocks nerve function. It is clinically used for infiltration anesthesia, nerve block anesthesia, epidural anesthesia, etc. Compared with procaine, its local anesthesia effect is remarkable. Tetracaine hydrochloride has been widely used in clinical practice. Tetracaine is in the ester-type local anesthetic family of medications. It is unclear if use during pregnancy is safe for the baby.
Definition
ChEBI: Tetracaine hydrochloride is a benzoate ester. It is a potent local anesthetic of the ester type used for surface and spinal anesthesia.
InChI:InChI=1/C15H24N2O2.ClH/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3;/h6-9,16H,4-5,10-12H2,1-3H3;1H
Tetracaine hydrochloride 136-47-0 Relevant articles
Preparation method of tetracaine hydrochloride
-
Paragraph 0026-0035, (2019/06/05)
The invention discloses a preparation method of tetracaine hydrochloride. The N,N-dimethyl ethanolamine is adopted to replace alcohol solvent in the aldehyde-amine reaction, solvent switching before a second esterification reaction is eliminated, and the possibility of generating impurities due to incomplete removal of the alcoholsolvent is eliminated, so that the content of a final product is effectively improved.
Method for preparing tetracaine hydrochloride
-
Paragraph 0039; 0040; 0042; 0043, (2019/01/21)
The invention discloses a method for preparing tetracaine hydrochloride. The method has the advantages that 1, the copper catalyst has smaller toxicity, is low in price and easily available while the copper catalyst is much safer, and the cost for raw materials and operation can be greatly reduced;2, the raw materials are easily available, the reaction has few steps, the intermediate reacting process can be easily controlled, few byproduct is generated, and large-scale production is easy to implement; and 3, the product has high purity and high total yield.
Preparation method of tetracaine hydrochloride
-
, (2017/04/22)
The invention relates to the technical field of preparation method of tetracaine hydrochloride. The preparation method comprises the preparation steps: carrying out a reaction of p-nitrobenzoyl chloride (2) and 2-dimethylamino-1-ethanol (3) to generate p-nitrobenzoic acid-2-dimethylamino ethyl (4), reducing the compound (3) to obtain p-aminobenzoic acid-2-dimethylamino ethyl (4), generating pontocaine (7) from a compound (5) and 1-bromobutane (6) under alkaline conditions, and finally carrying out a reaction of the pontocaine (7) with HCl to generate tetracaine hydrochloride (1).
136-47-0 Process route
-
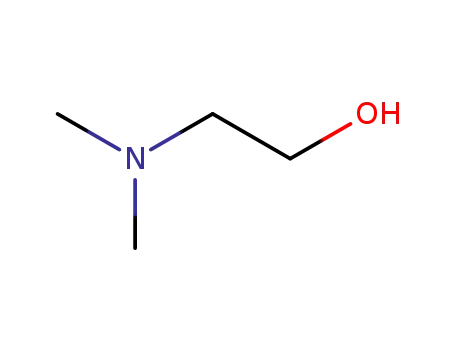
- 108-01-0
2-(N,N-dimethylamino)ethanol

-
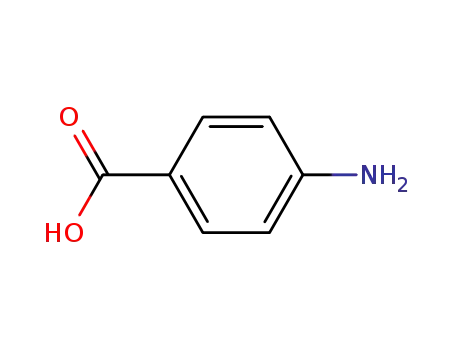
- 150-13-0,159246-81-8,8014-65-1
4-amino-benzoic acid

-
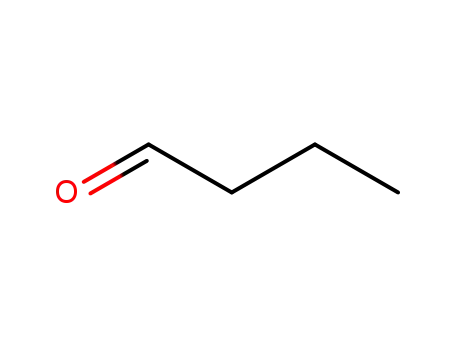
- 123-72-8
butyraldehyde

-
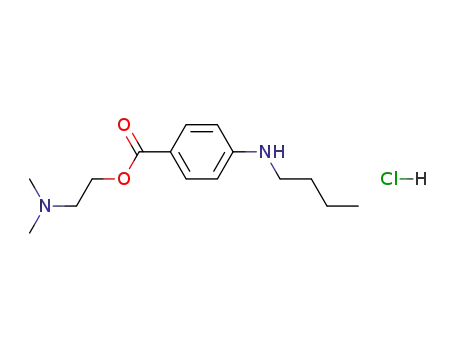
- 136-47-0,41585-83-5
tetracaine hydrochloride
| Conditions | Yield |
|---|---|
|
2-(N,N-dimethylamino)ethanol; 4-amino-benzoic acid; butyraldehyde; With hydrogen; at 40 - 45 ℃; for 3h; under 2250.23 Torr; Autoclave; Large scale;
With dmap; In 5,5-dimethyl-1,3-cyclohexadiene; for 4h; Reflux; Large scale;
With hydrogenchloride; In ethanol; tert-butyl methyl ether; water; at 45 ℃; pH=3 - 4; Reagent/catalyst; Temperature; Pressure; Solvent; Large scale;
|
75% |
-
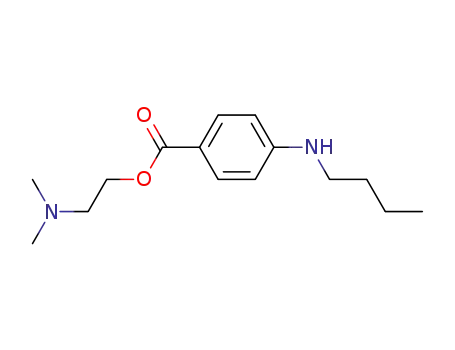
- 94-24-6
amethocaine

-

- 136-47-0,41585-83-5
tetracaine hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; ethyl acetate;
|
94% |
136-47-0 Upstream products
-
71134-92-4
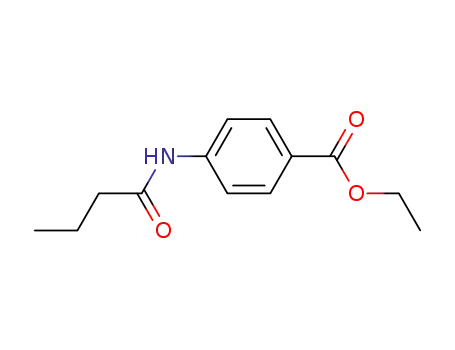
N-(4-ethoxycarbonylphenyl)butyramide
-
108-01-0

2-(N,N-dimethylamino)ethanol
-
94-32-6
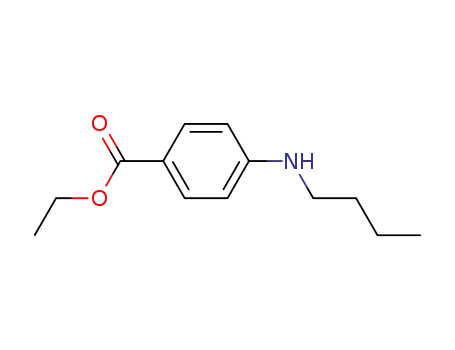
ethyl 4-(N-butylamino)benzoate
-
38152-22-6
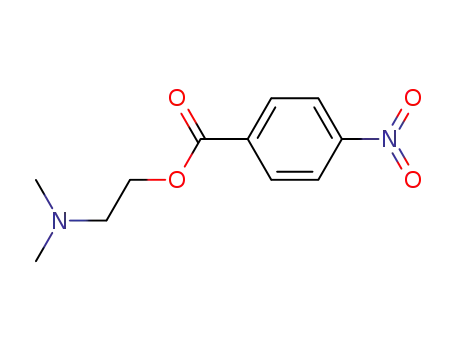
4-nitrobenzoic acid 2-(dimethylamino)ethyl ester
136-47-0 Downstream products
-
108-01-0

2-(N,N-dimethylamino)ethanol
-
4740-24-3
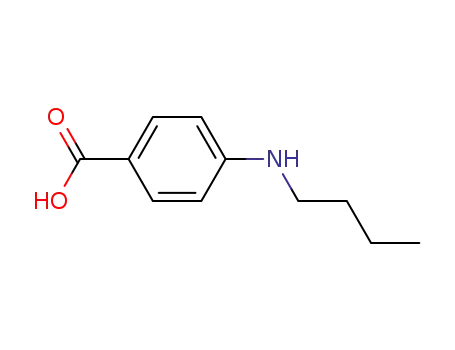
4-(N-butylamino)benzoic acid
-
1344028-77-8
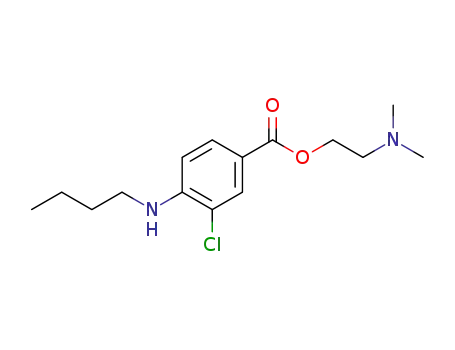
2-(dimethylamino)ethyl 4-(butylamino)-3-chlorobenzoate
-
150398-13-3
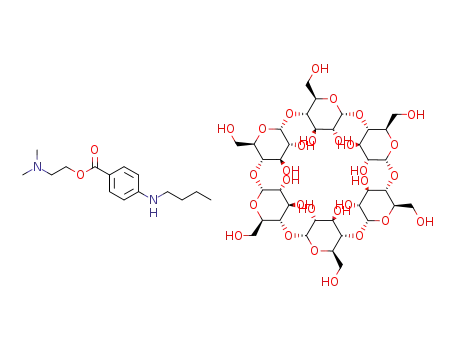
2-dimethylaminoethyl 4-(n-butylamino)benzoate α-cyclodextrin
Relevant Products
-
Sildenafil
CAS:139755-83-2
-
Tetraethylammonium bromide
CAS:71-91-0