139755-83-2
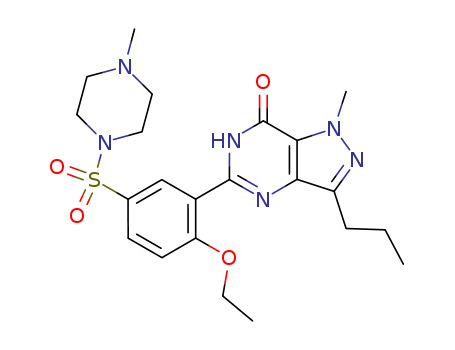
- Product Name:Sildenafil
- Molecular Formula:C22H30N6O4S
- Purity:99%
- Molecular Weight:
Product Details
Appearance:White crystalline powder
Sildenafil Intermediates 139755-83-2 Phenibut for sale
- Molecular Formula:C22H30N6O4S
- Molecular Weight:474.584
- Appearance/Colour:White crystalline powder
- Vapor Pressure:6.1E-18mmHg at 25°C
- Melting Point:187-189 ºC
- Refractive Index:1.664
- Boiling Point:672.4 ºC at 760 mmHg
- PKA:pKa 8.7 (Uncertain)
- Flash Point:360.5 ºC
- PSA:121.80000
- Density:1.39 g/cm3
- LogP:2.56750
139755-83-2 Usage
Sildenafil citrate is a white to off-white crystalline powder soluble in DMF, acetic acid and slightly soluble in methanol. Virility drug used to treat erectile dysfunction in men. Sildenafil (Viagra) is an oral medication called a phosphodiesterase-5 (PDE5) inhibitor approved for the treatment of pulmonary arterial hypertension (PAH) in World Health Organization (WHO) Group 1 patients. Sildenafil, the first US FDA-approved, oral phosphodiesterase type-5 inhibitor, has revolutionized the treatment of erectile dysfunction sine its approval in 1998. Since sildenafil is a potent inhibitor of cyclic guanosine monophosphate in the corpus cavernosum and therefore increases the penile response to sexual stimulation. About a quarter of men said that erection problems started between age 50 and 59, and 40% said they started between age 60 and 69. Sildenafil Citrate is an oral drug used to treat male erectile dysfunction and is a 5-phosphodiesterase inhibitor developed by the American Pfizer Pharmaceuticals to originally treat cardiovascular disease that was later discovered to improve patients'sex lives.
Overdose
Overdose information is limited. In studies with healthy volunteers, of single doses up to 800 mg, adverse events were similar to those seen at lower doses but incidence rates and severities were increased.
Originator
Pfizer (UK)
Definition
ChEBI: Sildenafil is a pyrazolo[4,3-d]pyrimidin-7-one having a methyl substituent at the 1-position, a propyl substituent at the 3-position and a 2-ethoxy-5-[(4-methylpiperazin-1-yl)sulfonyl]phenyl group at the 5-position. It has a role as a vasodilator agent and an EC 3.1.4.35 (3',5'-cyclic-GMP phosphodiesterase) inhibitor. It is a pyrazolopyrimidine, a member of piperazines and a sulfonamide.
Brand name
Viagra (Pfizer);Segurex.
InChI:InChI=1/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29)
139755-83-2 Relevant articles
Polymer-supported reagents for multi-step organic synthesis: Application to the synthesis of sildenafil
Baxendale, Ian R.,Ley, Steven V.
, p. 1983 - 1986 (2000)
Sildenafil 1 (Viagra(TM)), a well known and commercially important pharmaceutical drug, has been prepared using polymer-supported reagents in a multi-step, convergent process resulting in a clean and efficient preparation without the need for conventional purification methods. (C) 2000 Elsevier Science Ltd.
Molecular and crystal structure of sildenafil base
Stepanovs, Dmitrijs,Mishnev, Anatoly
, p. 491 - 494 (2012)
Sildenafil citrate monohydrate, well known as Viagra , is a drug for the treatment of erectile dysfunction. A comparison with the known crystal structures of sildenafil citrate monohydrate and sildenafil saccharinate is also presented.
α-Keto Acids as Triggers and Partners for the Synthesis of Quinazolinones, Quinoxalinones, Benzooxazinones, and Benzothiazoles in Water
Huang, Jian,Chen, Wei,Liang, Jiazhi,Yang, Qin,Fan, Yan,Chen, Mu-Wang,Peng, Yiyuan
, p. 14866 - 14882 (2021/10/25)
A general and efficient method for the synthesis of quinazolinones, quinoxalinones, benzooxazinones, and benzothiazoles from the reactions of α-keto acids with 2-aminobenzamides, benzene-1,2-diamines, 2-aminophenols, and 2-aminobenzenethiols, respectively, is described. More importantly, these reactions can be conducted on a mass scale, and the products can be easily purified through filtration and washing with ethanol (or crystallized).
Preparation method of sildenafil citrate
-
, (2021/04/07)
The invention discloses a preparation method of sildenafil. The preparation method disclosed by the invention is short in reaction time, greatly shortens the production period so as to reduce the production cost, and is simple in operation and suitable for large-scale production.
139755-83-2 Process route
-
![5-(5-Chlorosulphonyl-2-ethoxyphenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one](/upload/2023/1/694ad744-90f1-4ff2-82c9-b7c29781b9a7.png)
- 139756-22-2
5-(5-Chlorosulphonyl-2-ethoxyphenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one

-
- 34352-59-5,50398-09-9,51545-09-6
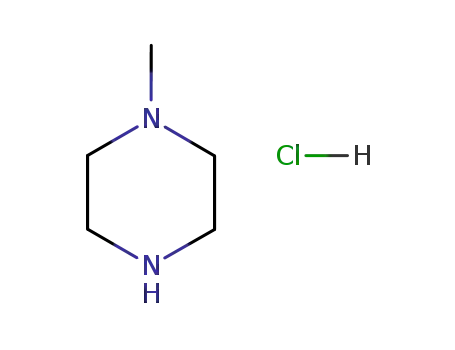
1-methylpiperazine hydrochloride

-
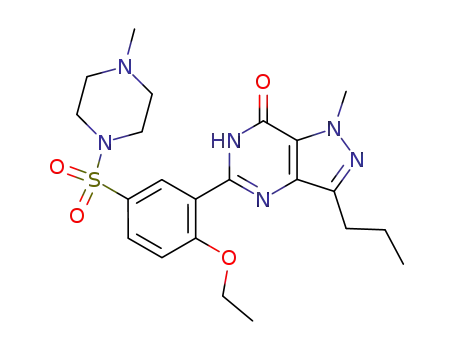
- 139755-83-2
viagra
| Conditions | Yield |
|---|---|
|
With triethylamine; N-ethyl-N,N-diisopropylamine; In water; at 60 ℃; for 1.2h;
|
91.8% |
-
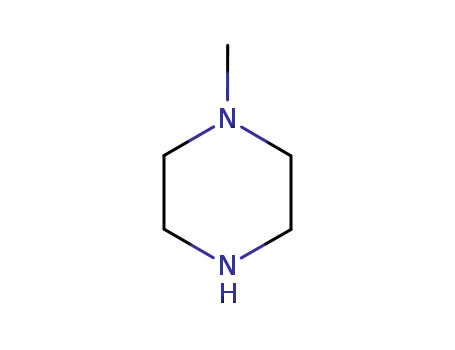
- 109-01-3
1-methyl-piperazine

-
![5-(5-Chlorosulphonyl-2-ethoxyphenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one](/upload/2023/1/694ad744-90f1-4ff2-82c9-b7c29781b9a7.png)
- 139756-22-2
5-(5-Chlorosulphonyl-2-ethoxyphenyl)-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one

-

- 139755-83-2
viagra
| Conditions | Yield |
|---|---|
|
With pyridine; In water; at 50 ℃; Reagent/catalyst; Temperature;
|
93.9% |
|
With triethylamine; In methanol; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
91% |
|
In acetone; at 20 ℃; for 3h; Sealed tube;
|
90% |
|
In tetrahydrofuran; at 20 ℃; for 3h; Sealed tube;
|
89% |
|
In tetrahydrofuran; at 20 ℃; for 3h;
|
89% |
|
With triethylamine; In isopropyl alcohol; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
86.1% |
|
With sodium hydroxide; In water; ethyl acetate; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
76.5% |
|
With sodium hydroxide; In water; toluene; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
75.7% |
|
With triethylamine; In acetonitrile; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
73% |
|
With triethylamine; In butanone; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
72% |
|
With sodium hydroxide; In methanol; water; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
70% |
|
With triethylamine; In ethanol; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
67.1% |
|
With triethylamine; In acetone; at 20 ℃; for 0.75h; Product distribution / selectivity;
|
62.3% |
|
With sodium hydroxide; In water; acetone; at 20 - 22 ℃; for 0.75 - 1h; Product distribution / selectivity;
|
57.5% |
|
In dichloromethane; at 20 - 30 ℃; Temperature; Solvent; Concentration;
|
58.8 g |
139755-83-2 Upstream products
-
109-01-3

1-methyl-piperazine
-
139756-21-1
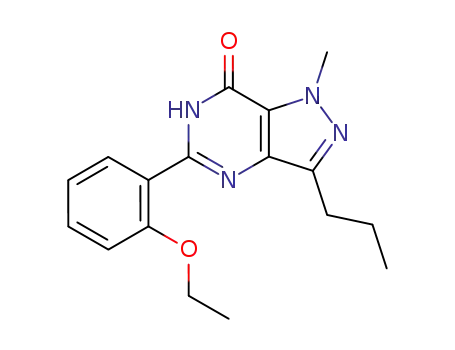
5-(2-ethoxy)-phenyl-1-methyl-3-n-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidine-7-one
-
200575-15-1
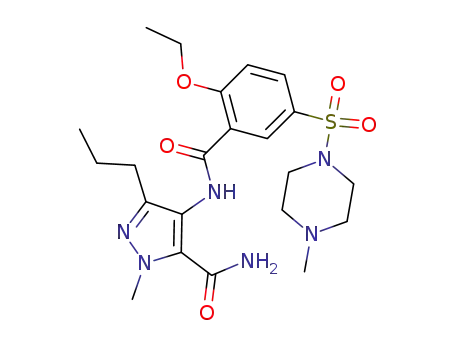
4-[2-ethoxy-5-(4-methyl-1-piperazinylsulphonyl)benzamido]-1-methyl-3-propyl-1H-pyrazole-5-carboxamide
-
960009-37-4
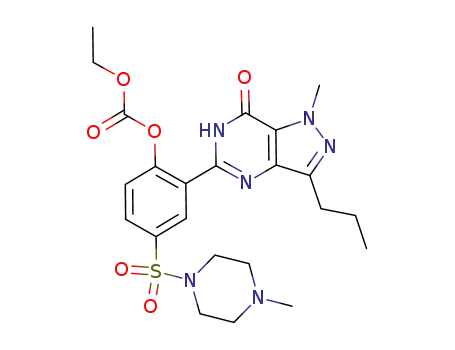
5-[2-ethoxycarbonyloxy-5-(4-methyl-piperazine-1-sulfonyl)-phenyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one
139755-83-2 Downstream products
-
252920-86-8
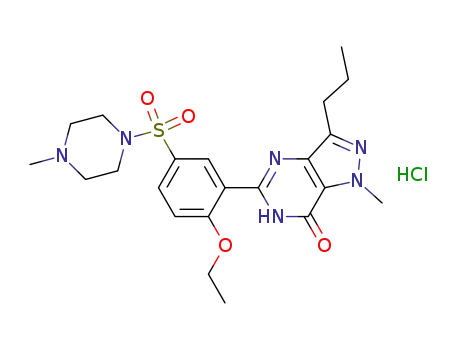
sildenafil hydrochloride
-
1094598-75-0
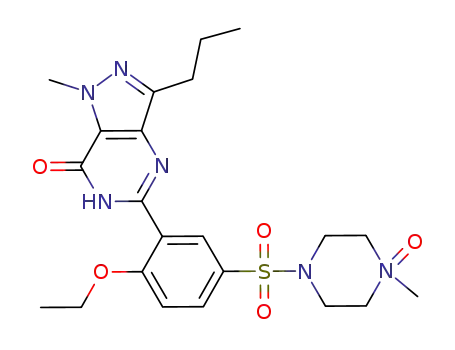
1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methyl-4-oxido-piperazine
-
1402916-50-0
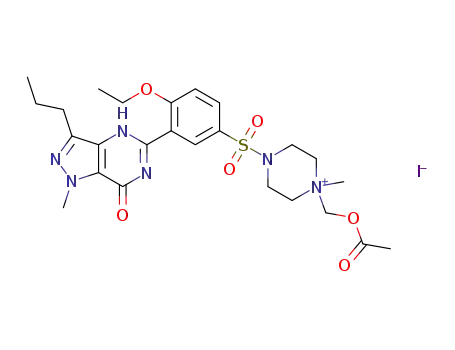
1-(acetoxymethyl)-4-((4-ethoxy-3-(1-methyl-7-oxo-3-propyl-4,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)phenyl)sulfonyl)-1-methylpiperazin-1-ium iodide
-
1485289-82-4
sildenafil succinate
Relevant Products
-
Sparteine sulfate anhydrous
CAS:299-39-8
-
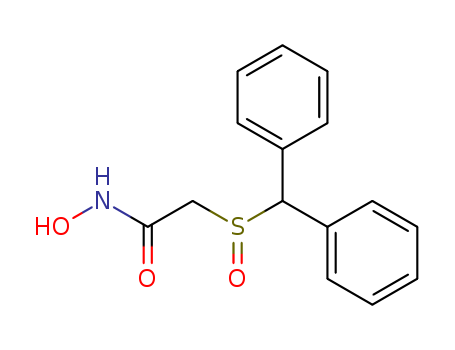
Acetamide,2-[(diphenylmethyl)sulfinyl]-N-hydroxy-
CAS:63547-13-7
-
Tetracaine hydrochloride
CAS:136-47-0