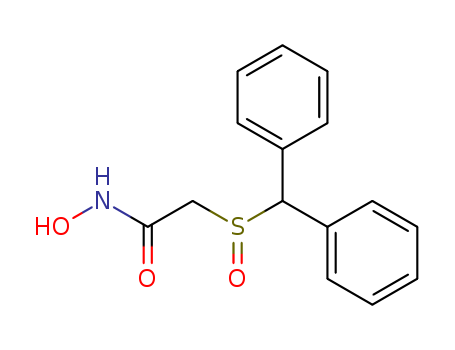
63547-13-7
- Product Name:Acetamide,2-[(diphenylmethyl)sulfinyl]-N-hydroxy-
- Molecular Formula:C15H15NO3S
- Purity:99%
- Molecular Weight:
Product Details
Appearance:White, hygroscopic powder.
Adrafinil 63547-13-7 Phenylpiracetam for sale, Good Supplier In China
- Molecular Formula:C15H15NO3S
- Molecular Weight:289.355
- Appearance/Colour:White, hygroscopic powder.
- Melting Point:150-160 °C
- Refractive Index:1.652
- PKA:8.22±0.20(Predicted)
- PSA:85.61000
- Density:1.342 g/cm3
- LogP:3.28670
Adrafinil 63547-13-7 Usage
Adrafinil, sold under the brand name Olmifon, is a drug designed for the treatment of narcolepsy by promoting an awakened state, and to treat alertness and neurological symptoms in the elderly. It is primarily metabolized in vivo to an active form, i.e. modafinil, 2-((diphenylmethyl)sulfinyl)acetamide. It has effects similar to modafinil, which is a prescription drug that is used to treat narcolepsy. Adrafinil, 2-((diphenylmethyl)sulfinyl)-N-hydroxyacetamide, is a drug designed for the treatment of narcolepsy by promoting an awakened state, and to treat alertness and neurological symptoms in the elderly. Adrafinil is a research chemical that’s touted for its potential applications as a nootropic.
InChI:InChI=1/C15H15NO3S/c17-14(16-18)11-20(19)15(12-7-3-1-4-8-12)13-9-5-2-6-10-13/h1-10,15,18H,11H2,(H,16,17)
63547-13-7 Relevant articles
Identification of Adrafinil and its Main Metabolite Modafinil in Human Hair. Self-Administration Study and Interpretation of an Authentic Case
Alice Ameline, Laurie Gheddar, Jean-Sébastien Raul, Pascal Kintz
Forensic Sciences Research, Volume 5, Issue 4, December 2020, Pages 322–326
Adrafinil and its metabolite, modafinil, were extracted from 20 mg decontaminated cut head hair, or cut beard hair, in presence of 20 ng of diazepam-d5, used as internal standard, after overnight incubation in 1 mL technical buffer solution at pH 4.01. Although adrafinil, modafinil and diazepam are chemically very different, the rationale for choosing diazepam-d5 is its use during the initial screening of each sample and therefore the possibility of direct confirmation of the extract with a more specific method (i.e. this proposed method).
Synthesis and determination of the absolute configuration of the enantiomers of modafinil
Prisinzano, Thomas,Podobinski, John,Tidgewell, Kevin,Luo, Min,Swenson, Dale
, p. 1053 - 1058 (2007/10/03)
The asymmetric synthesis of both enantiomers of modafinil, a unique CNS stimulant with a reduced abuse liability, is described. This approach effectively prepares modafinil on a multigram scale in several steps from benzhydrol. The described synthetic route has also been used to produce the more water soluble analogue, adrafinil. X-ray crystallographic analysis on (-)-(diphenylmethanesulfinyl)acetic acid has determined the absolute configuration to be R.
63547-13-7 Upstream products
-
63547-44-4
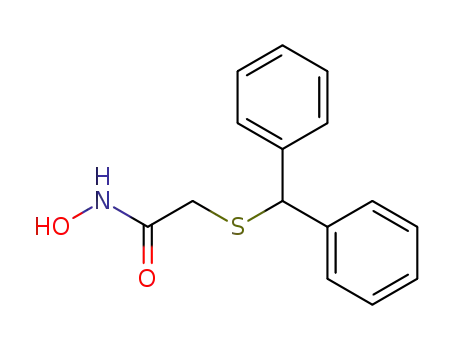
2-benzhydrylsulfanyl-N-hydroxyacetamide
-
91-01-0
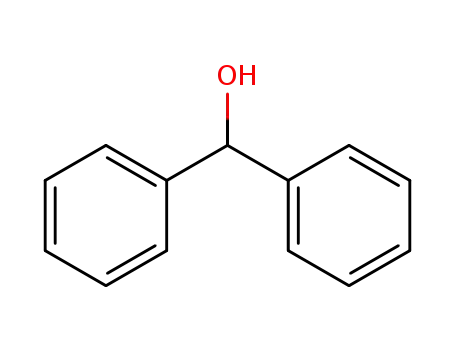
1,1-Diphenylmethanol
-
63547-22-8
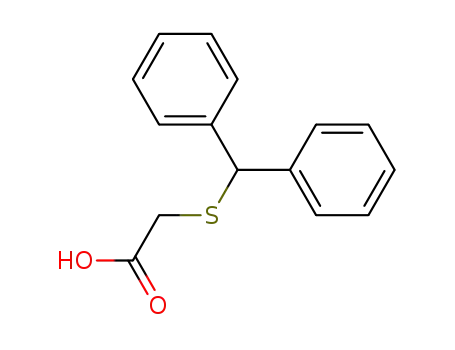
(benzhydrylthio)acetic acid
-
63547-23-9
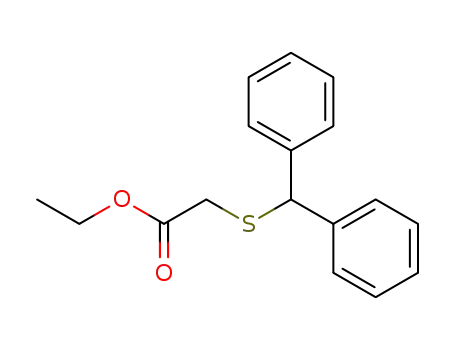
(diphenylmethyl)(ethyl acetate)sulfide
Relevant Products
-
Sparteine sulfate anhydrous
CAS:299-39-8
-
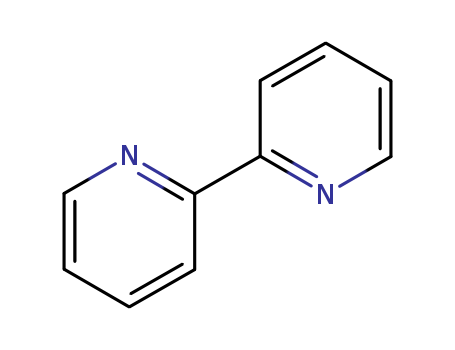
2,2'-Dipyridyl
CAS:366-18-7
-
Sildenafil
CAS:139755-83-2