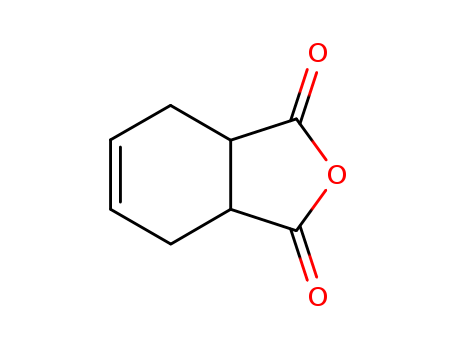
85-43-8
- Product Name:Tetrahydrophthalic Anhydride
- Molecular Formula:C8H8O3
- Purity:99%
- Molecular Weight:
Product Details
Appearance:WHITE CRYSTALLINE POWDER
Factory sells Tetrahydrophthalic Anhydride 85-43-8, in stock, Fast Delivery
- Molecular Formula:C8H8O3
- Molecular Weight:152.15
- Appearance/Colour:WHITE CRYSTALLINE POWDER
- Vapor Pressure:0.001mmHg at 25°C
- Melting Point:101-102 °C
- Refractive Index:1.529
- Boiling Point:305.6 °C at 760 mmHg
- Flash Point:148.2 °C
- PSA:43.37000
- Density:1.289 g/cm3
- LogP:0.65220
Tetrahydrophthalic Anhydride 85-43-8 Usage
Tetrahydrophthalic Anhydride (THPA), in the form of a white crystalline powder, is created from the reaction of butadiene with maleic anhydride. Highly toxic and a strong irritant to skin, eyes and mucous membranes. Corrosive to skin and metal. Tetrahydrophthalic anhydride (THPA) as curing agents were studied using dynamic differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and dynamic mechanical analysis (DMA). 1,2,3,6-Tetrahydrophthalic anhydride (THPA) can be used as: A curing agent for epoxides. A chemical modifier in the modification of polystyrene.
InChI:InChI=1/C8H8O3/c9-7-5-3-1-2-4-6(5)8(10)11-7/h1,3,5-6H,2,4H2
Shanghai Upbio Tech Co.,Ltd (Former Onchem (China)Co.,Ltd) is a comprehensive manufacturer and an international distribution of chemicals throughout the world. Our products mainly developing direction is "the best quality, the best service". In recent years, with the frequently international trading, which are sold well in Europe, America, Japan, Southeast Asia, more than 40 countries and regions, with excellent quality and competitive price.
85-43-8 Relevant articles
New Cyclic Imides and Quinazolin-2,4-diones Based on 1,2,3,6-Tetrahydrophthalic anhydride: Synthesis, Semiempirical Study and in vitro Evaluation
Ahmed M. Abo-Bakr, Mohamed Mobarak Taha, Antonous M. Mekhael, Mounir A. Mohamed
Page/Page column 4-6, (2018/10/11)
1,2,3,6-Tetrahydrophthalic anhydride (1) was used as a precursor for the synthesis of new cyclic imides by reacting with several reagents such as 2-aminothiazole, 4-aminopyridine, 2-aminobenzothiazole, cyanoacetic acid hydrazide, amino guanidine, 2,2-diaminomalononitrile, hexamethylenediamine or 2-(amino-ethyldisulfanyl)-ethylamine where the corresponding cyclic imides 2-9 were obtained respectively. The in vitro study of some selected imides and quinazolin-2,4-dinoes against two strains of bacteria possesses a high inhibition effect.
Tetrahydropiperic acid (THPA) conjugated cationic hybrid dipeptides as antimicrobial agents
Junaid ur Rahim, Gurpreet Singh, Sudha Shankar, Meenu Katoch & Rajkishor Rai
The Journal of Antibiotics volume 74, pages480–483 (2021)
The present work describes the synthesis of hybrid dipeptides H-Lys-Gpn-PEA, C1; H-Lys-β3,3AC6C-PEA, C2, and THPA conjugated dipeptides, THPA-Lys-Gpn-PEA, C3, and THPA-Lys-β3,3AC6C-PEA, C4. All the peptides were evaluated against both Gram-negative and Gram-positive bacterial strains. Among all, peptide C4 exhibited the most potent activity with MIC 1.56 μM against P. aeruginosa (MTCC 424) and S. aureus (MTCC 737). Further, time-kill kinetics, fluorescence assays, and scanning electron microscopy (SEM) studies were performed in order to understand the mechanism of action and efficacy of peptide C4, The fluorescence assays and SEM images demonstrated the bacterial killing through membrane disruption. The peptide C4 exhibited very low hemolytic activity with negligible cytotoxicity against normal human breast cell line FR2.
Formation of new substituted (1,3)oxazepine 1,5-diones via reaction of Exo-3,6-epoxy-1,2,3,6-tetrahydrophthalic Anhydride with Schiff’s bases
Mohammed G. Mukhlif; Omar J. Mahdi Al-Asafi; Ali K. Alywee
AIP Conference Proceedings 2457, 030005 (2023)
These Schiff's bases were treated in anhydrous 1,4-dioxane with Exo-3,6-epoxy-1,2,3,6-tetrahydrophthalic Anhydride under reflux in circumstances that gave substituted 1,3-oxazepine-1,5-dione. The products were isolated, purified and characterized by their melting points , FT-IR and 1HNMR spectra, which are found to be 1,3oxazepine 1,5-diones derivatives.
85-43-8 Upstream products
-
108-31-6
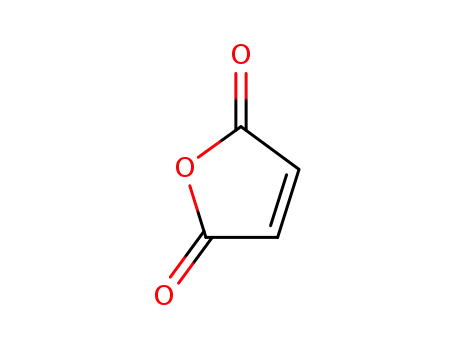
maleic anhydride
-
590-19-2
2,3-dimethylbutene
-
106-99-0
buta-1,3-diene
-
77-79-2
3-Sulfolene
85-43-8 Downstream products
-
19692-02-5
6-thiazol-2-ylcarbamoyl-cyclohex-3-enecarboxylic acid
-
39214-27-2
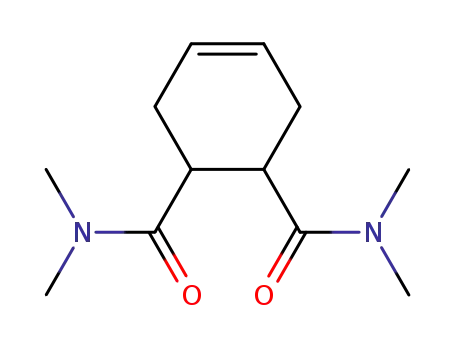
1,2,3,6-Tetrahydrophthalsaeure-bis-dimethylamid
-
40634-95-5
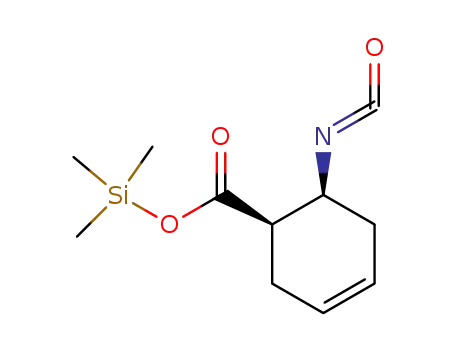
trimethylsilyl cis-2-isocyanato-4-cyclohex-1-ene carboxylate
-
1703-58-8
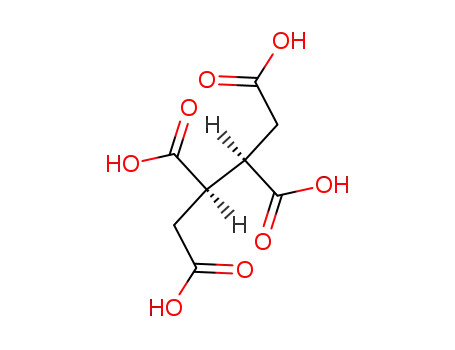
racem.-butane-1,2,3,4-tetracarboxylic acid
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
Anastrozole
CAS:120511-73-1
-
DL-Panthenol,Panthenol
CAS:16485-10-2