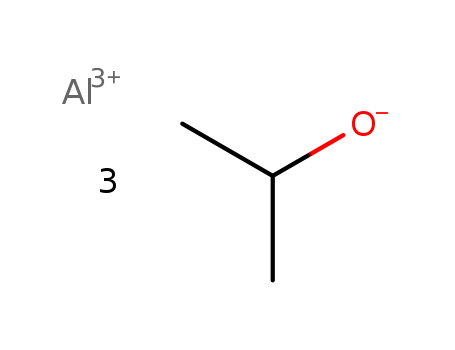
555-31-7
- Product Name:Aluminium isopropoxide
- Molecular Formula:C9H24O3Al
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white crystalline powder (may contain chunks)
Best price Reliable quality Aluminium isopropoxide 555-31-7 in bulk supply
- Molecular Formula:C9H24O3Al
- Molecular Weight:204.245
- Appearance/Colour:white crystalline powder (may contain chunks)
- Vapor Pressure:0.13 hPa (21 °C)
- Melting Point:129-136 °C
- Refractive Index:1.0346
- Boiling Point:125-130 °C (38 mmHg)
- Flash Point:46 °C
- PSA:27.69000
- Density:1.035 g/cm3
- LogP:2.62710
Best quality 555-31-7 good producer
Aluminum isopropoxide, also known as isopropyl aluminum oxide, abbreviated as AIP, is an oxide of aluminum. It is a useful reagent in organic synthesis. Aluminum isopropoxide on the market is mostly white translucent block, cylindrical small block solid or powder, soluble in isopropanol, ethanol, toluene, benzene, carbon tetrachloride, chloroform and other organic solvents (easy to dissolve when heated to 70 °C), decomposed when it meets water, has strong water absorption, and is easily decomposed into aluminum hydroxide and isopropanol.)Aluminium isopropoxide is usually described with the formula Al(O-i-Pr)3, where i-Pr is the isopropyl group (CH(CH3)2). This colourless solid is a useful reagent in organic synthesis. It is used in MPV reductions of ketones and aldehydes and the Oppenauer oxidation of secondary alcohols. Aluminum isopropoxide is synthesized by the reaction of isopropanol and aluminum under the catalysis of alumina. Raw material consumption quota: isopropyl alcohol 1255kg/t, aluminum 166kg/t.
Fire Hazard
The flash point of this compound is 26°C (79°F) (Bretzinger and Josten). It is less flammable than the sec- and tert-alkoxides of alkali metals. Ignition may occur when this compound is heated in moist air.It decomposes in water. The reaction is exothermic, producing isopropanol. It may decompose when heated to 250°C (482°F), producing highly flammable isopropyl ether.
Purification Methods
Redistil it under vacuum. Hygroscopic. [Robinson & Peak J Phys Chem 39 1127 1935, Beilstein 1 IV 1468.]
InChI:InChI=1/C3H8O.Al/c1-3(2)4;/h3-4H,1-2H3;/q;+3
555-31-7 Relevant articles
On the interaction of tetraethoxosilane with metal alkoxides: Sol-gel synthesis of alkaline-earth metal silicates
Suslova,Turova
, p. 1846 - 1854 (2006)
Physicochemical analyses (solubility method, conductometry, and IR spectroscopy) revealed no complex formation in M(OR)n-Si(OR) 4-ROH systems (M = Na, Ba, Al; R = Et, Pri), unlike in the systems containing alkoxides of two
Removal of Methylene Blue Dye from Waste Water Using Ionic Liquid Modified Aluminium Isopropoxide Under Ultrasonic Irradiations
Amanpreet Kaur*, Ashutosh Pathak** and Bhupinder Kaur*
Eco. Env. & Cons. 28 (May Suppl. Issue) : 2022; pp. (S220-S227)
It was found that the isotherm data had a good correlation with the Langmuir isotherm through analysing the experimental data by various models. The results showed that Methyltrioctylammonium chloride modified Aluminium isopropoxide was four times effective for the adsorption of MB dye under ultrasonication.
The state of Al(III) in alcohol solutions of aluminum alkoxide as probed by 27Al and 13C NMR and small-angle X-ray scattering
Fedotov,Molchanov,Zotov,Tuzikov
, p. 1621 - 1627 (2008)
Solutions of aluminum alkoxides obtained by interaction of aluminum metal with methyl, ethyl, and isopropyl alcohols were studied by 27Al and 13C NMR and small-angle X-ray scattering. The structure of polynuclear complexes was refined by comparison of their calculated dimensions with small-angle X-ray scattering data.
A Co-Precipitated α-Alumina (Al2O3) used as a TL-Micro Dosimeter
Said, Fouad,Tantawy, Hesham,El-Faramawy, Nabil,Abdel-Rahman, Mohamed A. E.
, p. 1354 - 1362 (2021)
This study describes the preparation method of alumina (Al2O3) as a type of thermoluminescence dosimeter (TLD) to monitor gamma photons (γ-rays) in abroad band of radioactive doses (0.5 : 2000 Gy). Henceforth, Co-precipitated α-alumina can be used as a TL-Micro dosimeter.
Preparation method of high-purity alkoxy aluminum
-
Paragraph 0020-0025; 0027-0034; 0039-0049; 0052-0059, (2021/12/07)
The invention provides a preparation method of high-purity alkoxy aluminum. According to the method, the reaction time of the fatty alcohol and the metal aluminum can be effectively shortened, and other impurities except impurities carried by the metal aluminum are not introduced.
Activity and stability studies of H-transfer reduction reactions of aldehydes and ketones over aluminium isopropoxide heterogenised catalysts
Atika Muhammad, Ammaru Ismaila, Bashir Jelani Usman, Graziano D Carmine and Carmine D'Agostino
RSC Adv., 2022, 12, 33970-33980
In this study, we investigate H-transfer reduction reactions of carbonyl compounds (aldehydes and ketones) catalysed by aluminium isopropoxide immobilised over mesoporous solid supports. The use of high surface area supports for the immobilisation is expected to enhance the dispersion of the aluminium isopropoxide catalyst.
Method for removing iron impurities in alkoxy aluminum
-
Paragraph 0038-0040; 0061-0064, (2019/11/20)
The invention discloses a method for removing iron impurities in alkoxy aluminum. According tothe method, an electrolytic method is used for removing the iron impurities in the alkoxy aluminum, operation is easy, and the effect is good.
555-31-7 Process route
-
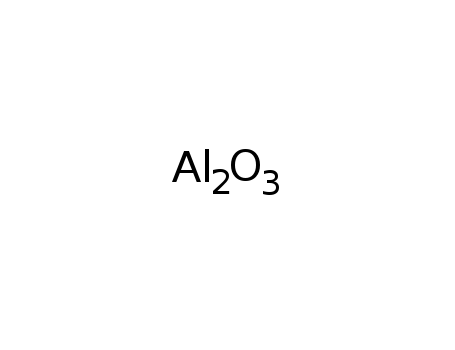
- 1333-84-2,1344-28-1
aluminum oxide

-
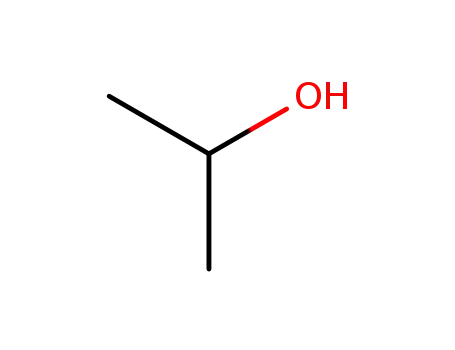
- 67-63-0,8013-70-5
isopropyl alcohol

-
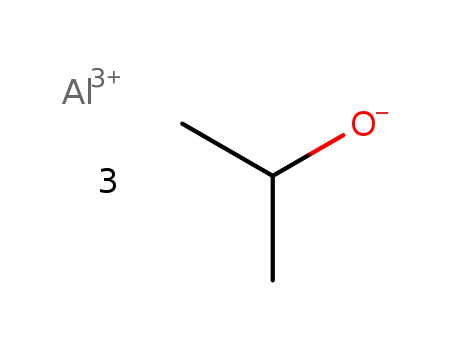
- 555-31-7
aluminum isopropoxide
| Conditions | Yield |
|---|---|
|
at 15 - 46 ℃; for 2.83333h; Temperature;
|
95% |
-

- 67-63-0,8013-70-5
isopropyl alcohol

-
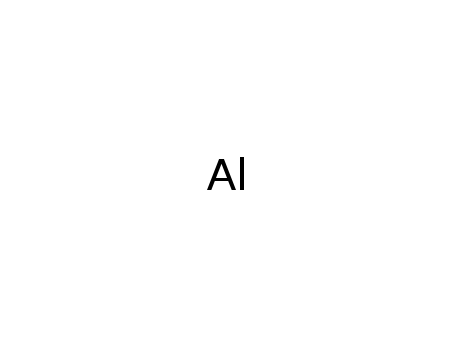
- 7429-90-5
aluminium

-

- 555-31-7
aluminum isopropoxide
| Conditions | Yield |
|---|---|
|
mercury dichloride; In isopropyl alcohol; direct reaction of Al metal with isopropanol in presence mercuric chloride as a catalyst;; distilled at 85°C/0.6mm; elem. anal.;;
|
|
|
elem. anal.;
|
|
|
With aluminium trichloride; In isopropyl alcohol; acetone; byproducts: H2; a round-bottomed flask loaded with Al and AlCl3, isopropanol added, mixt. heated on a H2O-bath to 81.5°C, isopropanol and acetone added when H2 release rate attained about 20 ml/min; Kinetics;
|
|
|
With hydrogenchloride; In isopropyl alcohol; acetone; byproducts: H2; a round-bottomed flask loaded with Al and HCl (concn. > 1.3 M), isopropanol added, mixt. heated on a H2O-bath to 81.5°C, isopropanol and acetone added when H2 release rate attained about 20 ml/min; Kinetics;
|
|
|
With iodine; In isopropyl alcohol; prepn. by dissolving of Al in dry alc. under heating and vigorous stirring; activated by introducting iodine into soln.;
|
|
|
mercury dichloride; In neat (no solvent); heating Al metal in an excess of 2-propanol, 82 °C, 5h;
|
|
|
mercury dichloride; In isopropyl alcohol; heating (excess of isopropanol, 10 h, 82°C);
|
|
|
In isopropyl alcohol; heating (82°C, 5 h);
|
|
|
mercury dichloride; according to "Textbook of Practical Organic Chemistry", 3rd. Ed., p. 883, from Al foil; distd. at reduced pressure at ca. 110°C;
|
|
|
In isopropyl alcohol; treatment of Al with alcohol in the presence of catalytic amts. of Al(OCH(CH3)2)3; distn. in vac. at 110 - 115°C and 1E-2 mmHg;
|
|
|
With mercury dichloride; for 9h; Reflux; Inert atmosphere;
|
|
|
With mercury dichloride; Inert atmosphere; Reflux;
|
|
|
With mercury dichloride; Reflux; Inert atmosphere;
|
|
|
With iodine; Reflux;
|
|
|
at 68 - 72 ℃; for 5h;
|
|
|
at 68 - 72 ℃; for 5.13h; Industrial scale;
|
|
|
for 4h;
|
|
|
at 82 ℃; Inert atmosphere; Sealed tube;
|
120.88 g |
555-31-7 Upstream products
-
67-63-0

isopropyl alcohol
-
97-93-8
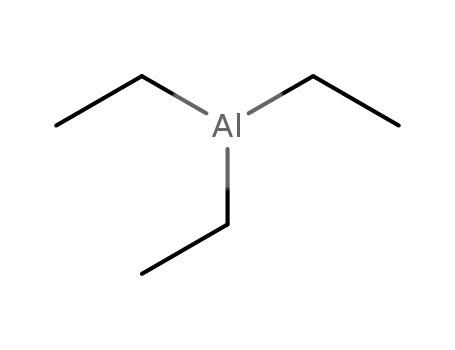
triethylaluminum
-
5804-85-3
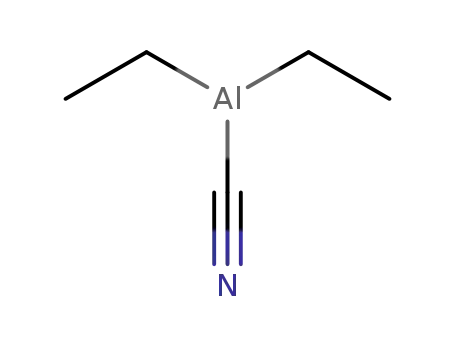
diethylaluminium cyanide
-
100-99-2
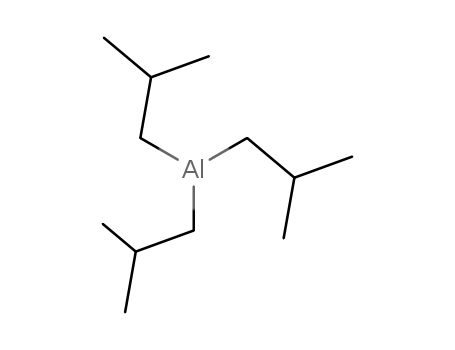
triisobutylaluminum
555-31-7 Downstream products
-
7152-24-1
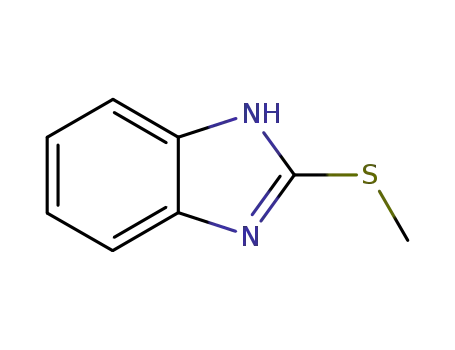
2-methylsulfanylbenzimidazole
-
4344-61-0
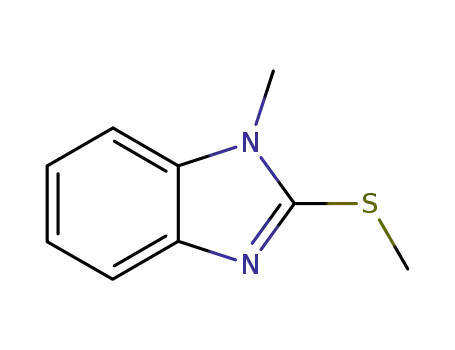
1-methyl-2-(methylthio)-1H-benzimidazole
-
3418-46-0
1,3-dimethyl-1,3-dihydro-benzoimidazole-2-thione
-
598-53-8
isopropyl methyl ether
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
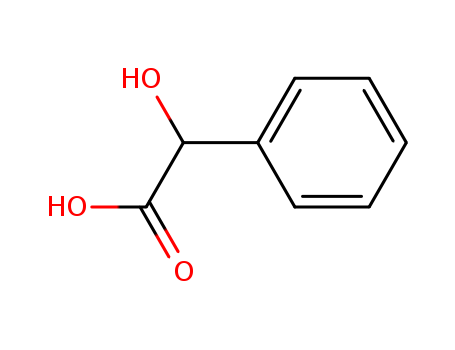
DL-Mandelic Acid
CAS:90-64-2
-
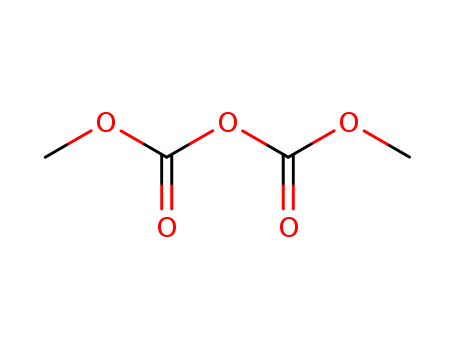
Dimethyl dicarbonate(DMDC)
CAS:4525-33-1