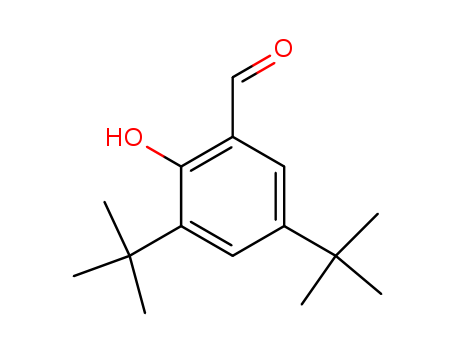
37942-07-7
- Product Name:3,5-Bis(1,1-dimethylethyl)-2-hydroxy-benzaldehyde
- Molecular Formula:C15H22O2
- Purity:99%
- Molecular Weight:
Product Details
Appearance:straw yellow to white crystal /powder
Global trade, Top quality 3,5-Bis(1,1-dimethylethyl)-2-hydroxy-benzaldehyde 37942-07-7 good supplier
- Molecular Formula:C15H22O2
- Molecular Weight:234.338
- Appearance/Colour:straw yellow to white crystal /powder
- Vapor Pressure:0.00266mmHg at 25°C
- Melting Point:59-61 °C(lit.)
- Refractive Index:1.527
- Boiling Point:277.6 °C at 760 mmHg
- PKA:9.78±0.23(Predicted)
- Flash Point:116.1 °C
- PSA:37.30000
- Density:1.006 g/cm3
- LogP:3.79970
3,5-Bis(1,1-dimethylethyl)-2-hydroxy-benzaldehyde 37942-07-7 Usage
3,5-Bis(1,1-dimethylethyl)-2-hydroxy-benzaldehyde is used in the synthesis of Mn(III)-salen complex, which is white to yellow powder or chunks or crystal or crystalline powder. It is a salicylaldehyde derivative with antibacterial activity used in the preparation nickel complexes. 3,5-Di-tert-butyl-2-hydroxybenzaldehyde is used in the synthesis of Mn(III)-salen complex and its diamino precursor 5,6-diamino-5,6-dideoxy-1,2-O-isopropylidene-3-O-methyl-β-L-idofuranose, chiral Schiff base ligand for an enantioselective copper-catalyzed addition of phenyl acetylene to imines, chiral oxazolidine ligand for the enantioselective addition of diethyl zinc to aldehydes and tin Schiff base complexes with histidine analogues. 3,5-Di-tert-butyl-2-hydroxybenzaldehyde was used in the synthesis ofMn(III)-salen complex and its diamino precursor 5,6-diamino-5,6-dideoxy-1,2-O-isopropylidene-3-O-methyl-β-L-idofuranosechiral Schiff base ligand for an enantioselective copper-catalyzed addition of phenylacetylene to imineschiral oxazolidine ligand for the enantioselective addition of diethylzinc to aldehydestin Schiff base complexes with histidine analogues
Synthesis Reference(s)
The Journal of Organic Chemistry, 59, p. 1939, 1994 DOI: 10.1021/jo00086a062Tetrahedron, 46, p. 793, 1990 DOI: 10.1016/S0040-4020(01)81362-4Tetrahedron Letters, 13, p. 4205, 1972 DOI: 10.1016/S0040-4039(01)94276-5
General Description
3,5-Di-tert-butyl-2-hydroxybenzaldehyde undergoes condensation reaction withmethyl-2-{N-(2′-aminoethane)}-amino-1-cyclopentenedithiocarboxylate to yield Schiff base ligandN,N-diethyl-2-methyl-1,4-phenylenediamine during the synthesis of copper(II) and cobalt(II) complexes of salicylaldimine
InChI:InChI=1/C15H22O2/c1-14(2,3)11-7-10(9-16)13(17)12(8-11)15(4,5)6/h7-9,17H,1-6H3
37942-07-7 Relevant articles
Quantum chemical insight into molecular structure, spectroscopic (FT‒IR, FT‒Raman, UV-vis, NMR), and molecular docking of 3,5-Bis(1,1-dimethylethyl)-2-hydroxy-benzaldehyde
S. J. Jenepha Mary, C. James
Chemical Data Collections Volume 29, October 2020, 100530
Antiviral activity of 3,5-Bis(1,1-dimethylethyl)-2-hydroxy-benzaldehyde has been carried out against influenza viral proteins of type A, type B, type C, and type D. Molecular docking simulations shows it has a good binding affinity toward influenza type D virus.
Cobalt(II) phenoxy-imine complexes in radical polymerization of vinyl acetate: The interplay of catalytic chain transfer and controlled/living radical polymerization
Chen, Yi-Hao,Chen, Shih-Ji,Li, Jia-Qi,Wu, Zhenqiang,Lee, Gene-Hsiang,Liu, Yi-Hung,Cheng, Wei-Ting,Yeh, Chen-Yu,Peng, Chi-How
, (2019)
A series of cobalt(II) phenoxy-imine complexes (CoII(FI)2) have been synthesized to mediate the radical polymerization of vinyl acetate (VAc) and methyl acrylate (MA) to evaluate the influence of chelating atoms and configuration to the control of polymerization. Associated with the results of polymerization mediated by other cobalt complexes, this study implied that the configuration and spin state of cobalt complexes were more critical than the chelating atoms to the control behavior of radical polymerization.
Manganese and Iron Complexes with Malonic Acid Bis[2-(3,5-di-tert-butyl-2-hydroxybenzyl)hydrazide]
P. A. Fatullaeva
Russian Journal of General Chemistry volume 89, pages1649–1652 (2019)
The structure of the complexes has been studied by IR and electronic spectroscopy and X-ray analysis. Malonic acid bis[(2-hydroxy-3,5-di-tert-butylbenzylidene)-hydrazide] has been shown to form dinuclear complexes with manganese and iron ions, in which ferromagnetic interaction between the metal ions is observed.
Synthesis of 6-tert-octyl and 6,8-ditert-butyl coumarins, two coumarins of biological interest
Arroyo, P.,Darouch, M.,Lisboa, E.,Miranda, A.,Zárraga, M.
, p. 5220 - 5222 (2021/07/12)
In this study, the synthesis of new coumarins with aliphatic chains is discussed. The incorporation of the 6-tert-octyl and 6,8-ditert-butyl chains into a coumarin structure from alkylphenols, allows obtaining hydrophobic coumarins with good yields. These coumarins can be potential modulators of TRPV1 receptors. Synthesis and spectroscopic data of these new coumarins are analyzed.
37942-07-7 Process route
-
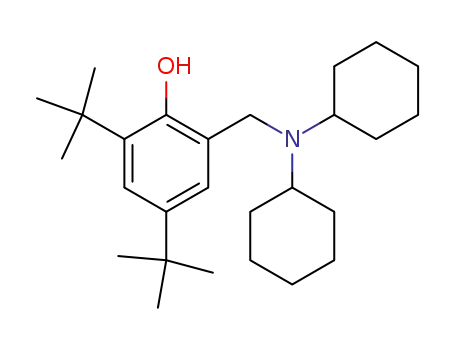
-
2,4-di-tert-butyl-6-((dicyclohexylamino)methyl)phenol

-
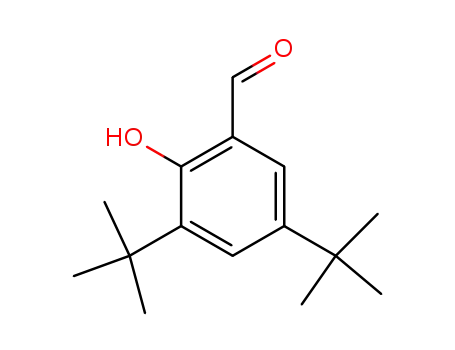
- 37942-07-7
3,5-di-tert-butyl-2-hydroxybenzaldehyde

-
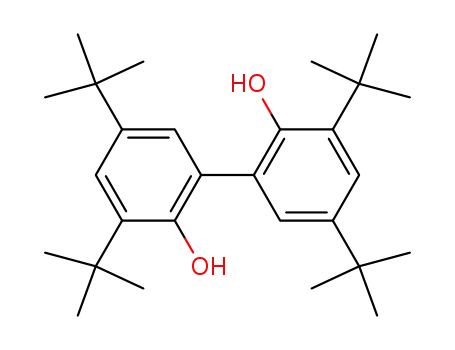
- 6390-69-8
3,3',5,5'-tetra(tert-butyl)biphenyl-2,2'-diol
| Conditions | Yield |
|---|---|
|
With manganese triacetate; In acetic acid; for 2h;
|
95% |
-
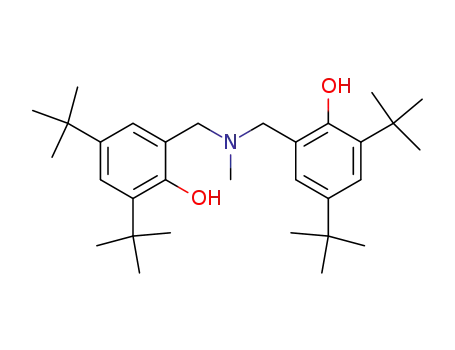
- 120695-70-7
Methylimino-2,2'-dimethylene-bis-(4,6-di-tert-butyl-phenol)

-

- 37942-07-7
3,5-di-tert-butyl-2-hydroxybenzaldehyde

-

- 6390-69-8
3,3',5,5'-tetra(tert-butyl)biphenyl-2,2'-diol

-
![N-methyl-2,3-dihydro-1H-6,8-bis(1,1-dimethylethyl)benz[1,2-e][1,3]oxazine](/upload/2023/1/2c7a0d53-a5d3-4cf8-b675-03d763e4fbc3.png)
- 128203-42-9
N-methyl-2,3-dihydro-1H-6,8-bis(1,1-dimethylethyl)benz[1,2-e][1,3]oxazine
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; potassium hexacyanoferrate(III); In methanol; water;
|
15% 20% 50% |
37942-07-7 Upstream products
-
98-54-4
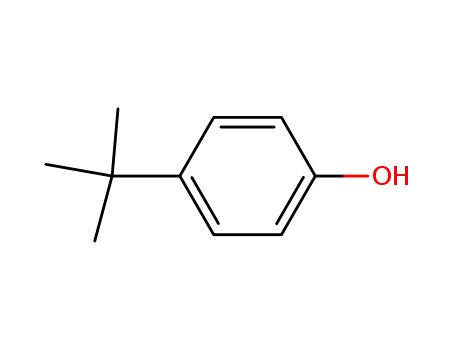
para-tert-butylphenol
-
96-76-4
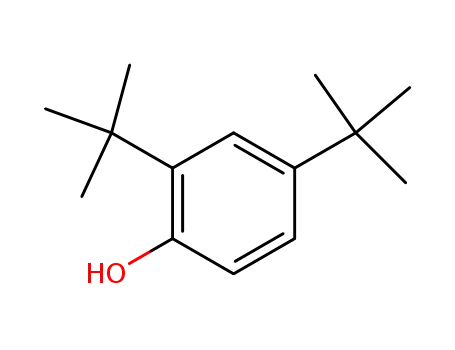
2,4-di-tert-Butylphenol
-
100-97-0
hexamethylenetetramine
-
67-56-1
methanol
37942-07-7 Downstream products
-
103627-63-0
3-[1-(3,5-Di-tert-butyl-2-hydroxy-phenyl)-meth-(Z)-ylidene]-dihydro-furan-2-one
-
155052-31-6
2,4-di-tert-butyl-6-({[(1S)-1-(hydroxymethyl)-2-methylpropyl]imino}methyl)phenol
-
151433-25-9
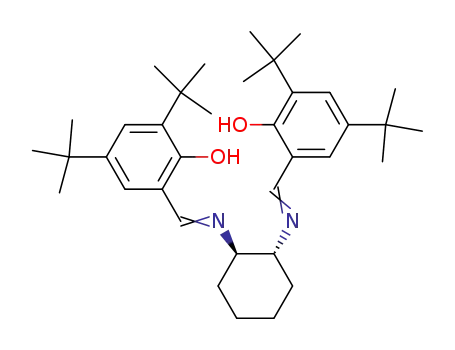
(R,R)-N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine
-
864066-01-3
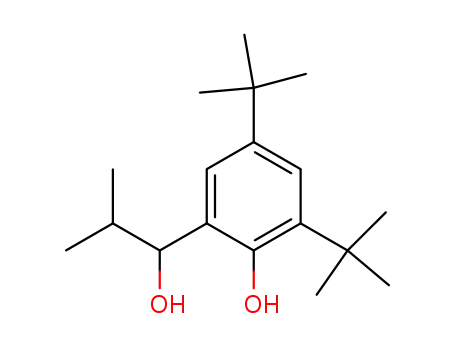
2,4-di-tert-butyl-6-(1-hydroxy-2-methylpropyl)phenol
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
Ibuprofen
CAS:15687-27-1
-
Mucins, gastric
CAS:84082-64-4