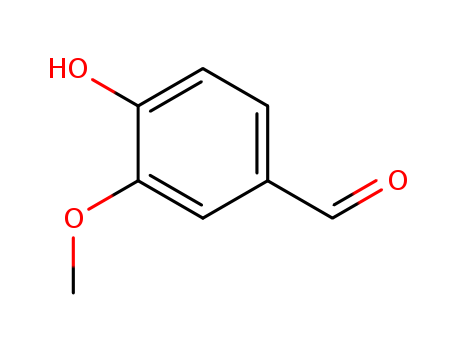
Product Details
Appearance:White or very slightly yellow needles
Vanillin superior manufacturer supply High Purity 121-33-5 in stock
- Molecular Formula:C8H8O3
- Molecular Weight:152.15
- Appearance/Colour:White or very slightly yellow needles
- Vapor Pressure:>0.01 mm Hg ( 25 °C)
- Melting Point:81-84 °C
- Refractive Index:Health: 1; Flammability: 1; Reactivity: 0
- Boiling Point:282.6 °C at 760 mmHg
- PKA:pKa 7.396±0.004(H2O I = 0.00 t = 25.0±1.0) (Reliable)
- Flash Point:117.6 °C
- PSA:46.53000
- Density:1.231 g/cm3
- LogP:1.21330
Vanillin Usage
Vanillin is commonly known as vanilla powder, cloud Nepal with powder, vanilla extract, is extracted from the Rutaceae vanilla bean, is a kind of important spices, is one of synthetic fragrances yield the largest varieties, mixing chocolate, ice cream, chewing gum, pastry and tobacco essence of important raw materials. Vanilla is the main natural flavoring agent used in industries such as pharmaceuticals, food, flavoring, and fragrance, in which vanillin is the major component. The use of vanillin depends largely on its type or source. Natural and identical-natural vanillin are used only in consumables (food, beverages, confectionery, and pharmaceuticals), while synthetic vanillin is frequently used in the polymer industry and in detergents, balms, and perfumes. Moreover, only natural-labeled vanillin is approved for use in the food industry by most food safety control authorities worldwide.
Toxicity
LD50 orally in rats, guinea pigs: 1580, 1400 mg/kg (Jenner)
Limited use
FEMA (mg/kg): soft drinks 63; cold 95; candy 200; baking food 220; pudding class 120, chewing gum, 270; chocolate 970; decorating layer 150; margarine 0.20; syrup 330~20000. According to the provisions of FAO/WHO: The allowable amount is 70mg/kg for fast food, of canned baby foods and cereals (1992).
Definition
ChEBI: A member of the class of benzaldehydes carrying methoxy and hydroxy substituents at positions 3 and 4 respectively.
Fire Hazard
Flash point data for Vanillin are not available, however Vanillin is probably combustible.
Flammability and Explosibility
Nonflammable
InChI:InChI:1S/C8H8O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-5,10H,1H3
121-33-5 Relevant articles
Current Status, Challenges, and Prospects for the Biological Production of Vanillin
Wankui Jiang ,Xiaoyue Chen ,Yifan Feng ,Jingxiang Sun ,Yujia Jiang ,Wenming Zhang ,Fengxue Xin and Min Jiang
Fermentation 2023, 9(4), 389
In this article, vanillin synthesis pathways and related genes reported in the literature were summarized. An overview of the latest progress and future development in the biological production of vanillin from lignin and ferulic acid was provided, and several production process optimization technologies were compared.
Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions
C Iannuzzi, M Liccardo, I Sirangelo
Int. J. Mol. Sci. 2023, 24(3), 1817
In conclusion, vanillin seems to be a promising, accessible, and novel neuroprotective agent and it could be used as functional food and a food supplement for the prevention of neurological disorders.
Source identification of vanillin in sesame oil by HPLC-MS/MS
Mengying Wang , Yuepeng Lu , Yong Yang , Jiahao Yu , Yechao Chen , Fengqin Tu , Jing Hou , Zong Yang , Xiaoming Jiang
Food Control Volume 143, January 2023, 109283
In this research, a convenient HPLC-MS/MS analytical method was developed for the determination of vanillin, methyl vanillin and ethyl vanillin in sesame seed.
121-33-5 Process route
-
alkaline lignin
-
alkaline lignin

-
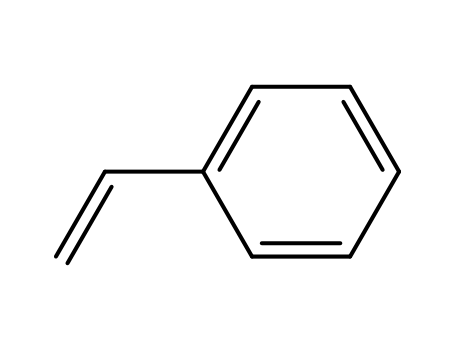
- 100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene

-
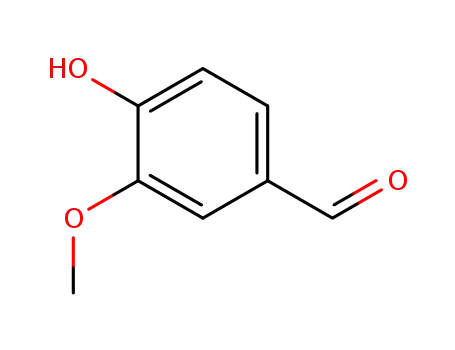
- 121-33-5,8014-42-4
vanillin

-
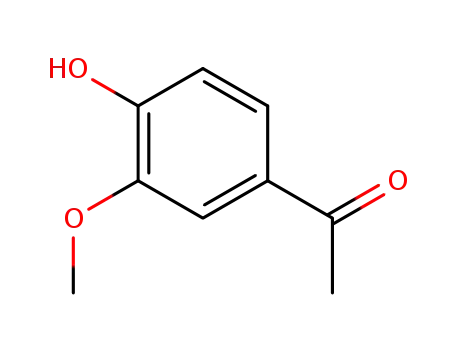
- 498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone
| Conditions | Yield |
|---|---|
|
With prolinium tetrachloromanganate(II); ethylammonium nitrate (EAN); at 35 ℃; for 6h;
|
12% |
-
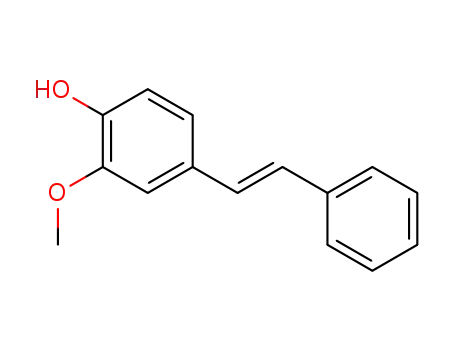
- 3391-75-1,18486-72-1
2-methoxy-4-((E)-phenylethenyl)phenol

-
![2-Methoxy-4-[7-methoxy-2-phenyl-5-((E)-styryl)-2,3-dihydro-benzofuran-3-yl]-phenol](/upload/2023/1/d4465257-fac4-4742-a54c-70484c8e29b4.png)
- 122917-29-7
2-Methoxy-4-[7-methoxy-2-phenyl-5-((E)-styryl)-2,3-dihydro-benzofuran-3-yl]-phenol

-
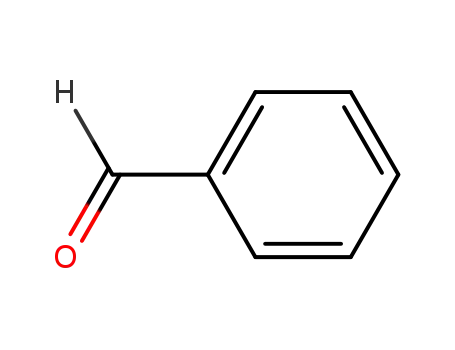
- 100-52-7
benzaldehyde

-

- 121-33-5,8014-42-4
vanillin
| Conditions | Yield |
|---|---|
|
With oxygen; Co(SalMe)Py; In 1,2-dichloro-ethane; at 25 ℃; for 5h;
|
17% 17% 55% |
121-33-5 Upstream products
-
67-56-1
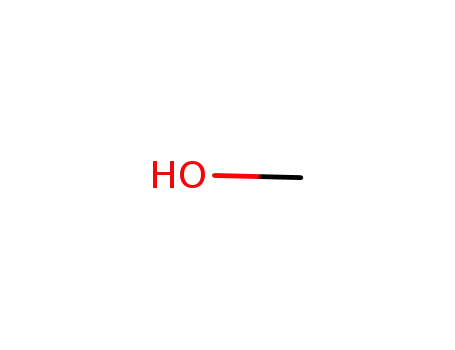
methanol
-
2973-78-6
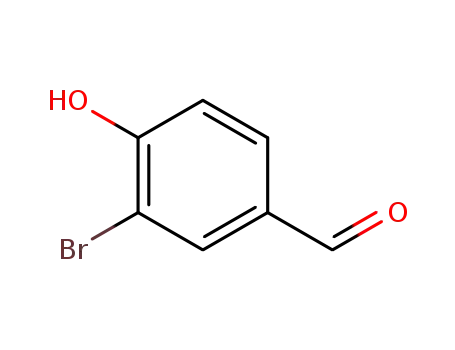
3-bromo-4-hydroxybenzylaldehyde
-
50597-26-7
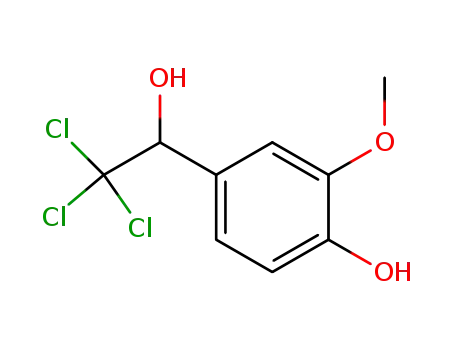
2,2,2-trichloro-1-(4-hydroxy-3-methoxy-phenyl)-ethanol
-
124-41-4
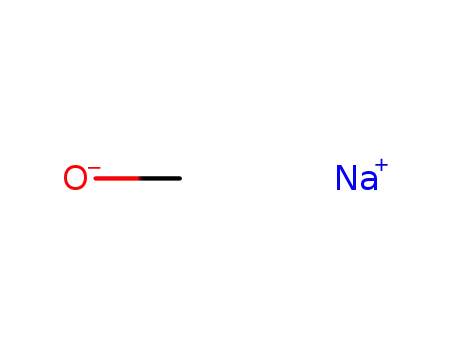
sodium methylate
121-33-5 Downstream products
-
141186-15-4
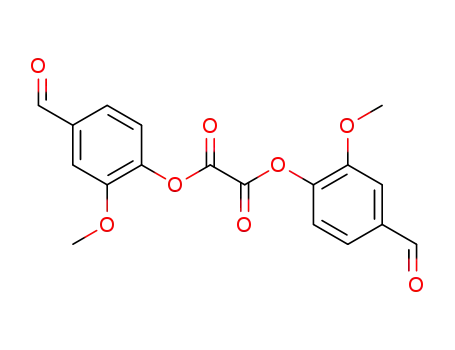
oxalic acid bis-(4-formyl-2-methoxy-phenyl ester)
-
314754-67-1
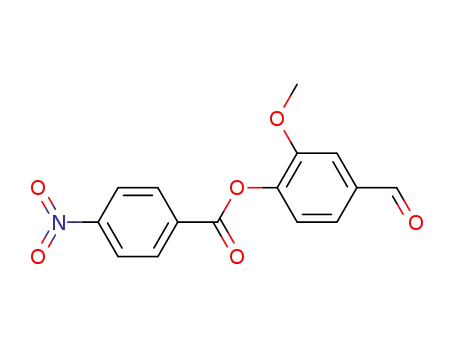
4‐formyl‐2‐methoxyphenyl 4‐nitrobenzoate
-
46995-88-4
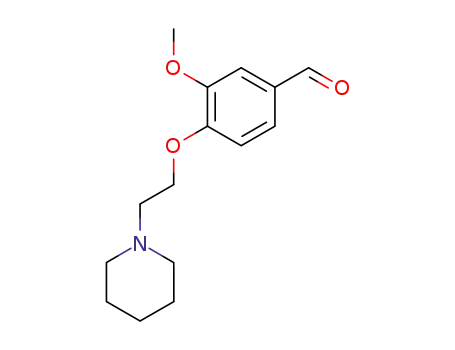
3-methoxy-4-[2-(piperidine-1-yl)ethoxy]-benzaldehyde
-
5431-89-0
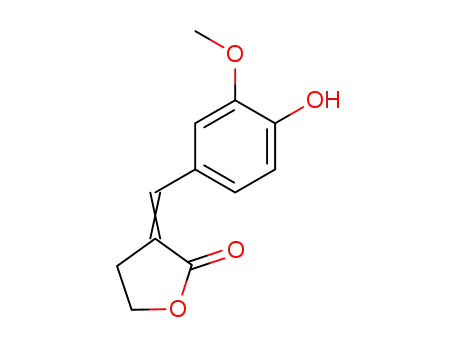
3-((Ξ)-vanillylidene)-dihydro-furan-2-one
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
D-Mannitol
CAS:69-65-8
-
Tranexamic acid
CAS:1197-18-8