119356-77-3
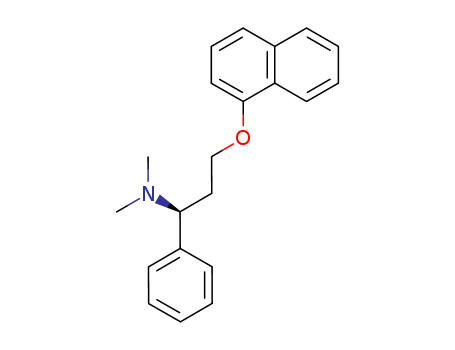
- Product Name:Dapoxetinehydrochloride
- Molecular Formula:C21H23NO
- Purity:99%
- Molecular Weight:
Product Details
Appearance:White to off-white crystalline powder
Hot sale, Dapoxetine HCL 119356-77-3 Intermediates for sale
- Molecular Formula:C21H23NO
- Molecular Weight:305.42
- Appearance/Colour:White to off-white crystalline powder
- Vapor Pressure:1.91E-08mmHg at 25°C
- Refractive Index:1.607
- Boiling Point:454.4 °C at 760 mmHg
- PKA:8.38±0.50(Predicted)
- Flash Point:132.6 °C
- PSA:12.47000
- Density:1.081 g/cm3
- LogP:4.91160
Shanghai Upbio Tech Co.,Ltd (Former Onchem (China)Co.,Ltd) have our own GMP factory with R&D centure.and cleaning workshop , and some main products produced by our own lab and R&D centure.besides we have established very good cooperation and business relations with hundreds of Chinese qualified manufacturers by long-term business intercourse.
Dapoxetine HCL Usage
Premature ejaculation (PE) is the most common male sexual dysfunction. Dapoxetine hydrochloride, belonging to a class of drugs known as selective serotonin reuptake inhibitors or, was the first drug originally approved for the on-demand treatment of men with PE. Dapoxetine, marketed as Priligy, among others, is a selective serotonin reuptake inhibitor used for the treatment of premature ejaculation in men 18–64 years old. Dapoxetine hydrochloride is the newest SSRI launched last year for the oral on-demand treatment of PE in men between 18 and 64 years of age.
InChI:InChI=1/C21H23NO/c1-22(2)21(18-9-4-3-5-10-18)14-15-23-20-13-12-17-8-6-7-11-19(17)16-20/h3-13,16,21H,14-15H2,1-2H3
119356-77-3 Relevant articles
Synthesis method of dapoxetine
-
Paragraph 0040; 0045-0046; 0051; 0055-0056; 0061, (2022/01/12)
The synthesis method of dapoxetine of the present invention is inexpensive and easy to obtain raw materials, does not use toxic and dangerous reagents, will not react to the phenomenon of aggregation spray, the process is simple, suitable for industrial production.
Enantioconvergent Cu-Catalyzed Radical C-N Coupling of Racemic Secondary Alkyl Halides to Access α-Chiral Primary Amines
Cheng, Jiang-Tao,Dong, Xiao-Yang,Gu, Qiang-Shuai,Li, Zhong-Liang,Liu, Juan,Liu, Xin-Yuan,Luan, Cheng,Wang, Fu-Li,Wang, Li-Lei,Yang, Ning-Yuan,Zhang, Yu-Feng
supporting information, p. 15413 - 15419 (2021/09/30)
α-Chiral alkyl primary amines are virtually universal synthetic precursors for all other α-chiral N-containing compounds ubiquitous in biological, pharmaceutical, and material sciences. These results shine light on the potential of enantioconvergent radical cross-coupling as a general chiral carbon-heteroatom formation strategy.
Synthetic method of dapoxetine and intermediate thereof
-
Paragraph 0110-0113, (2020/03/09)
The invention discloses a synthetic method of dapoxetine and its intermediate, i.e., (S)-3-(tert-butyloxycarbonyl)amino-3-phenylpropanol as shown in a formula 5 which is described in the specification. The synthetic method of dapoxetine and the intermediate thereof has the characteristics of usage of cheap and easily available raw materials, high yield and low cost, and is more beneficial to industrial production.
RP-HPLC with Time Programmed Fluorescence Detection for Quantitation of Avanafil and Dapoxetine Hydrochloride: Application to Pharmaceutical Dosage Form and Biological Fluid
M. Hegazy,A. Kessiba,M. Abdelkawy &A. E. El-Gindy
Journal of Liquid Chromatography & Related Technologies Volume 38, 2015 - Issue 18
Avanafil (AVN) was recently co-formulated with dapoxetine HCl (DAP) for treatment of erectile dysfunction and premature ejaculation. Sensitive and simple reversed-phase (RP) high-performance liquid chromatographic method (HPLC) was developed and validated for their simultaneous determination using tadalafil (TAD) as an internal standard. Isocratic separation was achieved within run time of only 7.0 min on Eclipse C18 column (150 mm × 4.6 mm, 5 µm particle size) using a mobile phase composed of acetonitrile: 0.15% triethylamine (40:60, v/v) at pH = 4.0 adjusted with o-phosphoric acid.
119356-77-3 Upstream products
-
90-15-3
α-naphthol
-
82769-75-3
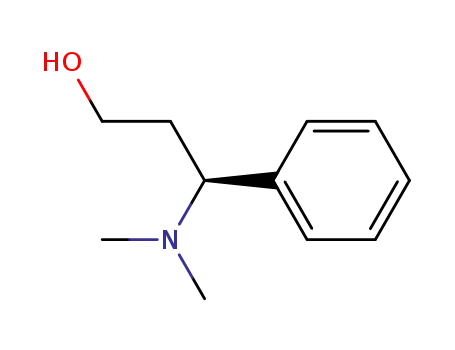
(S)-(+)-3-(dimethylamino)-3-phenyl-1-propanol
-
147199-39-1
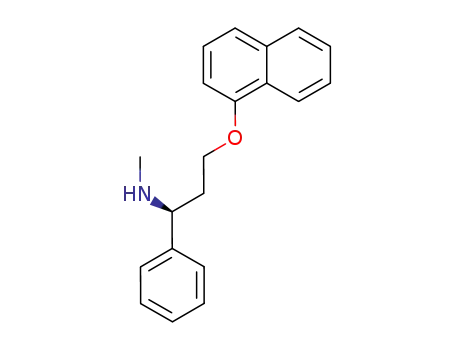
Desmethyldapoxetine
-
50-00-0
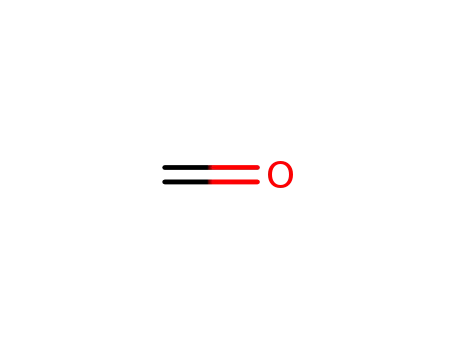
formaldehyd
119356-77-3 Downstream products
-
1365438-38-5
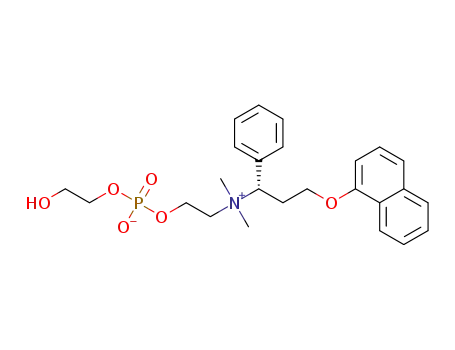
(S)-2-(dimethyl(3-(naphthalen-1-yloxy)-1-phenylpropyl)ammonio)ethyl (2-hydroxyethyl) phosphate
-
1071929-03-7
N,N-dimethylamino-3-(naphthyl-1-oxy)-1-phenylprop-1-amine hydrochloride
Relevant Products
-
Cyclopropane, isocyanato- 4747-72-2
CAS:4747-72-2
-
Progesterone
CAS:57-83-0
-
Metformin
CAS:657-24-9