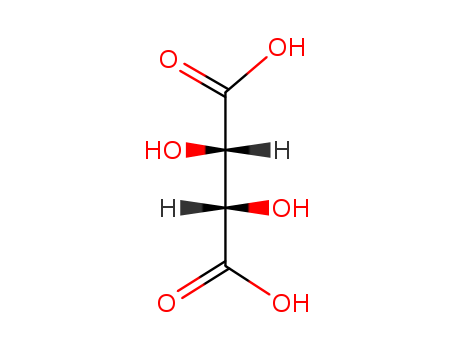
147-71-7
- Product Name:D-(-)-Tartaric acid
- Molecular Formula:C4H6O6
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white crystals
Hot Sale 147-71-7 D-(-)-Tartaric acid supplier, good producer
- Molecular Formula:C4H6O6
- Molecular Weight:150.088
- Appearance/Colour:white crystals
- Vapor Pressure:4.93E-08mmHg at 25°C
- Melting Point:172-174 °C(lit.)
- Refractive Index:-12.5 ° (C=5, H2O)
- Boiling Point:399.3 °C at 760 mmHg
- PKA:3.0, 4.4(at 25℃)
- Flash Point:209.4 °C
- PSA:115.06000
- Density:1.886 g/cm3
- LogP:-2.12260
D-(-)-Tartaric acid 147-71-7 Usage
D-(-)-Tartaric acid is a polycrystalline solid, widely used as an acidizing agent for beverages and other foods, and this use is similar to citric acid. Tartaric Acid is an acidulant that occurs naturally in grapes. It is hygroscopic and rapidly soluble, with a solubility of 150 g in 100 ml of distilled water at 25°c. D-tartaric acid is the D-enantiomer of tartaric acid. It has a role as an Escherichia coli metabolite. It is a conjugate acid of a D-tartrate(1-). D-(-)-Tartaric acid is used as a resolving agent in organic synthesis. It is used as a precursor for the preparation of its ester derivatives like D-tartaric acid diethyl ester, D-tartaric acid dimethyl ester and D-tartaric acid diiso-propyl ester. It finds application in the synthesis of chiral aziridine derivative, a common intermediate for the preparation of hydroxyethylamine class HIV protease inhibitors viz. as saquinavir, amprenavir and nelfinavir. It is widely used in the food industry as a beer foaming agent, for food acidity regulations and as a flavoring agent. The difference between L tartaric acid and D-tartaric acid is that they have an OH group on the right hand side in d- configuration, while on the left hand side in l- configuration.
Definition
ChEBI: The D-enantiomer of tartaric acid.
InChI:InChI=1/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/p-2/t1-,2-/m0/s1
147-71-7 Relevant articles
Enhancing the Stability of Nicotine via Crystallization Using Enantiopure Tartaric Acid Salt Formers
Devin J. Angevine, Kristine Joy Camacho, Xiaotong Zhang, Javid Rzayev, and Jason B. Benedict
ACS Omega, April 18, 2023
Two nicotinium tartrate salts were synthesized; one utilizing d-(−)-tartaric acid and one utilizing l-(+)-tartaric acid. Single crystals of (S)-nicotinium bis-l-(+)-tartrate dihydrate (henceforth referred to as the l-tartrate salt) suitable for X-ray diff.
Structural insight into the catalytic mechanism of a cis-epoxysuccinate hydrolase producing enantiomerically pure d(-)-tartaric acid
Dong, Sheng,Liu, Xi,Cui, Gu-Zhen,Cui, Qiu,Wang, Xinquan,Feng, Yingang
, p. 8482 - 8485 (2018/08/04)
Crystal structure determination and mutagenesis analysis of a cis-epoxysuccinate hydrolase which produces enantiomerically pure d(-)-tartaric acids revealed a zinc ion and essential residues in the stereoselective mechanism for the catalytic reaction of the small mirror symmetric substrate.
147-71-7 Process route
-
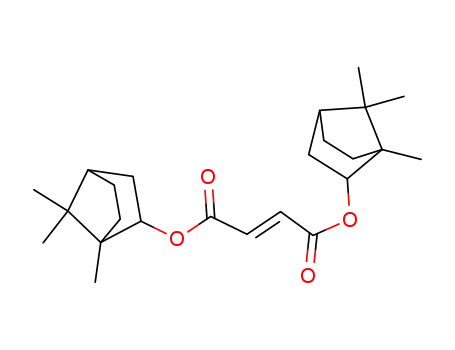
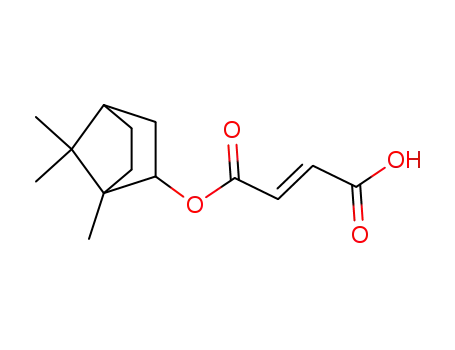
- 77734-68-0,78392-58-2,78392-59-3
di-1-bornyl fumarate

-
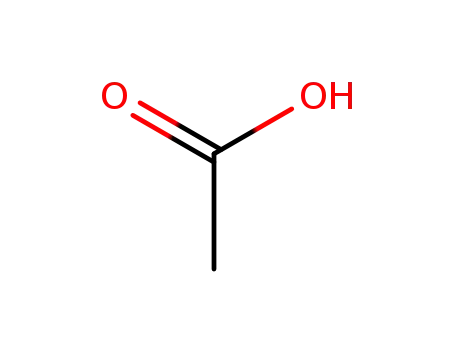
- 64-19-7,77671-22-8
acetic acid

-
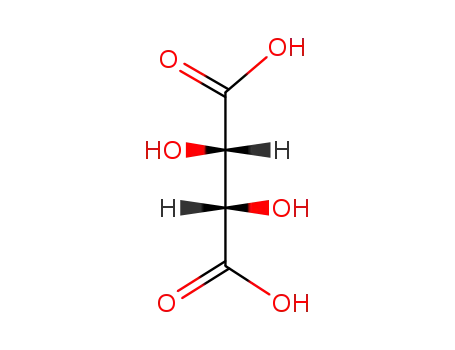
- 147-71-7,138508-61-9
D-tartaric acid

-
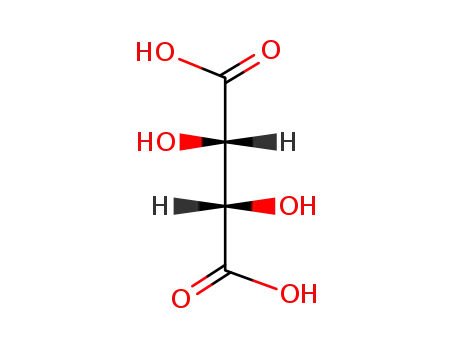
- 133-37-9,138508-61-9
DL-tartaric acid
| Conditions | Yield |
|---|---|
|
fumaric acid di-l-bornyl ester;
|
-
- 54685-70-0,117894-84-5
fumaric acid monobornyl ester

-

- 147-71-7,138508-61-9
D-tartaric acid

-

- 133-37-9,138508-61-9
DL-tartaric acid
| Conditions | Yield |
|---|---|
|
fumaric acid mono-l-bornyl ester; Verseifung des Reaktionsproduktes;
|
147-71-7 Upstream products
-
74-90-8

hydrogen cyanide
-
453-17-8
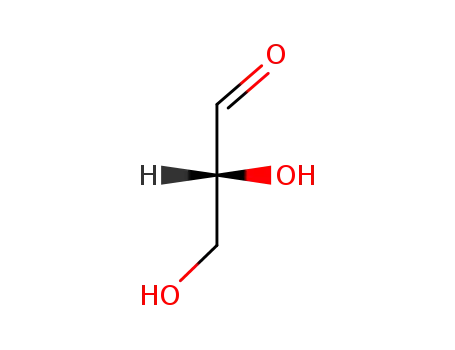
D-Glyceraldehyde
-
2418-52-2
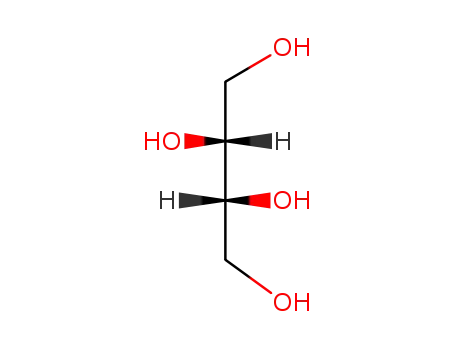
D-threitol
-
1113-60-6
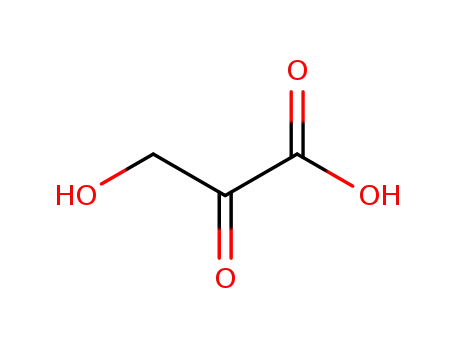
3-hydroxy-2-oxopropionic acid
147-71-7 Downstream products
-
1730-91-2
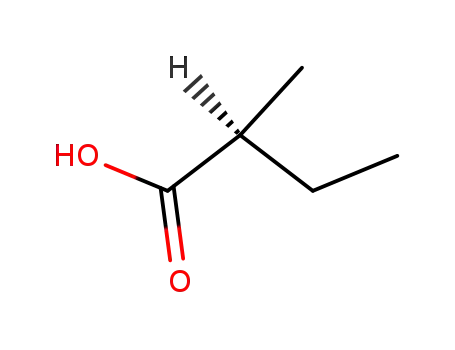
(S)-2-Methylbutyric acid
-
133-37-9

DL-tartaric acid
-
72842-25-2
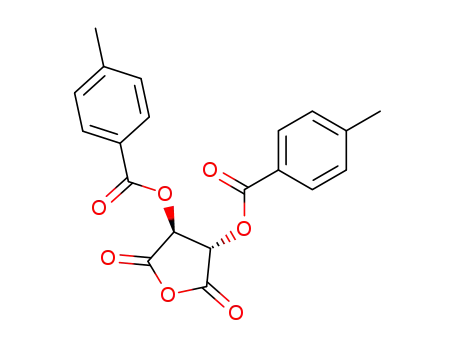
(3S,4S)-2,5-dioxotetrahydrofuran-3,4-diyl bis(4-methylbenzoate)
-
37577-07-4
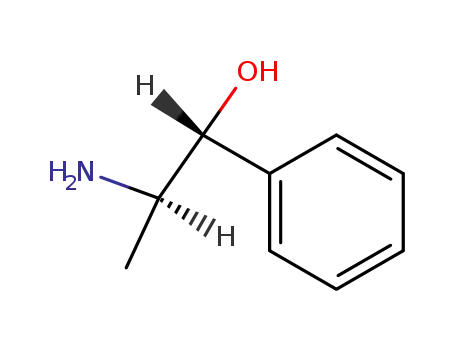
Norpseudoephedrine
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
Metronidazole
CAS:443-48-1
-
Aspirin
CAS:50-78-2