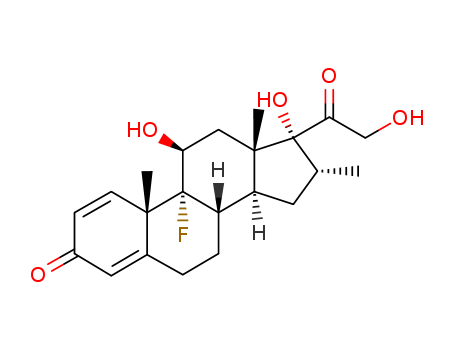
Product Details
Appearance:White crystalline solid
Dexamethasone Wholesaler, 50-02-2 in stock, Good Supplier In China
- Molecular Formula:C22H29FO5
- Molecular Weight:392.468
- Appearance/Colour:White crystalline solid
- Vapor Pressure:2.81E-15mmHg at 25°C
- Melting Point:262-264 °C(lit.)
- Refractive Index:76 ° (C=1, Dioxane)
- Boiling Point:568.2 °C at 760 mmHg
- PKA:12.13±0.70(Predicted)
- Flash Point:297.5 °C
- PSA:94.83000
- Density:1.32 g/cm3
- LogP:1.89570
50-02-2 Usage
Dexamethasone is an Anti-inflammatory glucocorticoid that is used to treat inflammatory and autoimmune conditions such as rheumatoid arthritis and bronchospasm. It is useful to study apoptosis, cell signaling pathways and gene expression. Dexamethasone is a artificially synthetic glucocorticoid, belonging to a long-term glucocorticoid drugs. It is a corticosteroid known as a glucocorticoid. The activity of dexamethasone, as measured by glycogen deposition, is 20 times greater than that of hydrocortisone. A judicious selection of the available tests may be necessary to obtain an accurate diagnosis in patients with Cushing’s syndrome.
Isomeric SMILES: C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@@]4([C@]3([C@H](C[C@@]2([C@]1(C(=O)CO)O)C)O)F)C InChIKey: UREBDLICKHMUKA-CXSFZGCWSA-N InChI: InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1
50-02-2 Relevant articles
Antigen-Drug Conjugates as a Novel Therapeutic Class for the Treatment of Antigen-Specific Autoimmune Disorders
Pickens, Chad J.,Christopher, Matthew A.,Leon, Martin A.,Pressnall, Melissa M.,Johnson, Stephanie N.,Thati, Sharadvi,Sullivan, Bradley P.,Berkland, Cory
, p. 2452 - 2461 (2019)
Multiple sclerosis represents the world's most common cause of neurological disability in young people and is attributed to a loss of immune tolerance toward proteins of the myelin sheath. These results highlight the benefits of co-delivery of autoantigens with immunosuppressant drugs as AgDCs for the treatment of autoimmune diseases.
Thalidomide and dexamethasone combination for refractory multiple myeloma
M.A. Dimopoulos,K Zervas,G Kouvatseas,E Galani,V Grigoraki,C Kiamouris,E Vervessou,E Samantas,C Papadimitriou,O Economou
,Annals of Oncology 2001 Jul;
All patients were resistant to standard chemotherapy, 77% were resistant to dexamethasone-based regimens and 32% had previously received high-dose therapy. RESULTS: On an intention-to-treat basis twenty-four patients (55%) achieved a partial response with a median time to response of 1.3 months.
A 17, 21 - double-hydroxy steroid derivatives of synthetic method
-
, (2018/04/02)
The invention relates to a method of preparing 17, 21-double-hydroxyl steroid derivatives by using 6, 9-substituted silyl steroid enol ether compound I as an initiator.
50-02-2 Process route
-
-
C23H31FO4

-
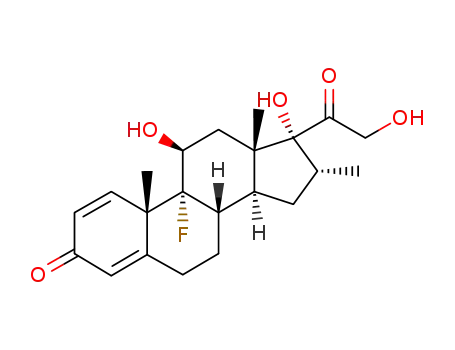
- 50-02-2
dexamethasone
| Conditions | Yield |
|---|---|
|
C23H31FO4; In ethyl acetate; at 0 - 10 ℃;
With hydrogenchloride; In water; at 30 - 35 ℃; for 1h;
|
40% |
-
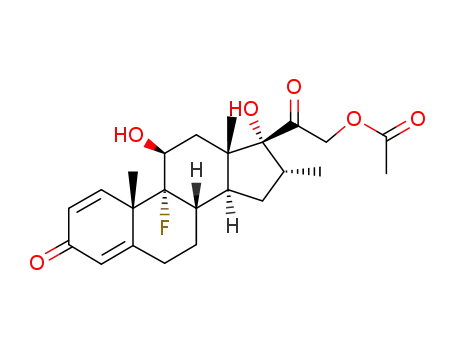
- 1177-87-3
betamethasone

-

- 50-02-2
dexamethasone
| Conditions | Yield |
|---|---|
|
In ethanol; at 24 - 26 ℃; for 96h; Penicillium decumbens ATCC 10436, potato dextrose broth;
|
5% |
|
With methanol; sodium carbonate; at 20 ℃; for 0.166667h; Temperature;
|
50-02-2 Upstream products
-
1966-25-2
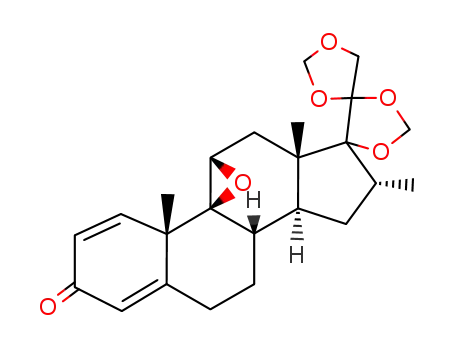
16α-methyl-9β,11β-oxido-17α,20;20,21-bismethylenedioxy-pregna-1,4-diene-3-one
-
1177-87-3

betamethasone
-
13209-41-1
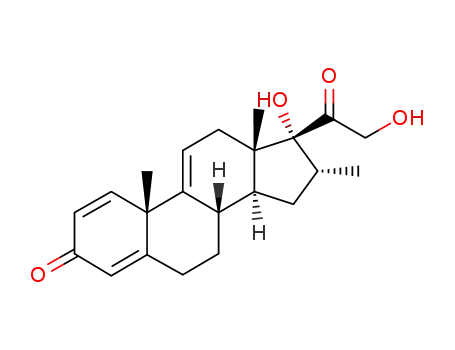
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one
-
14518-56-0
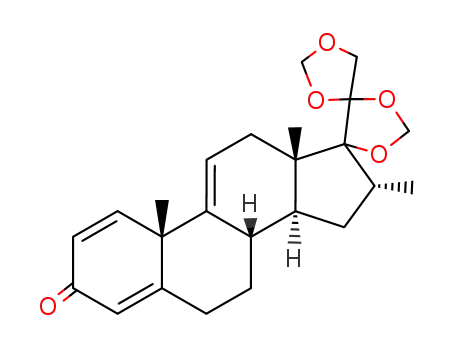
16α-methyl-17α,20;20,21-bismethylenedioxypregn-1,4,9(11)-triene-3-one
50-02-2 Downstream products
-
2265-22-7
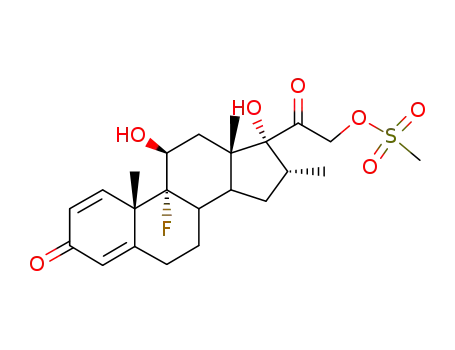
dexamethasone-21-mesylate
-
25122-41-2
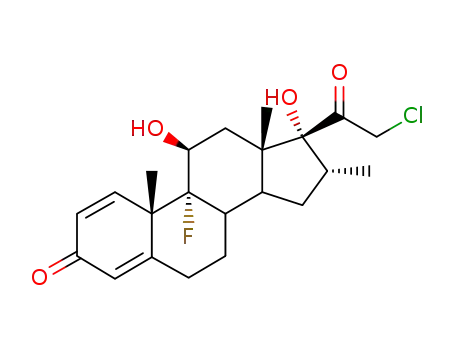
dexamethasone-21-chloride
-
1177-87-3
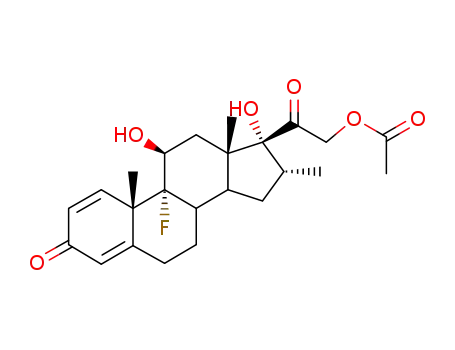
Dexamethasonacetat
-
1893-66-9
dexamethasone 21-iodoacetate
Relevant Products
-
High quality Ticagrelor 99%
CAS:274693-27-5
-
Testosterone Enanthate
CAS:315-37-7
-
Cetilistat
CAS:282526-98-1