Product Details
Appearance:white to yellow crystalline powder
Ergosterol is a natural product which is the most abundant sterol in fungal cell membranes, we offer best quality Ergosterol 57-87-4, in bulk supply.
Best quality Ergosterol 57-87-4 for sale, in bulk supply
- Molecular Formula:C28H44O
- Molecular Weight:396.657
- Appearance/Colour:white to yellow crystalline powder
- Vapor Pressure:3.7E-12mmHg at 25°C
- Melting Point:156-158 °C(lit.)
- Refractive Index:-112.5 ° (C=1, THF)
- Boiling Point:501.5 °C at 760 mmHg
- PKA:14.91±0.70(Predicted)
- Flash Point:216.3 °C
- PSA:20.23000
- Density:1 g/cm3
- LogP:7.33080
Ergosterol 57-87-4 Usage
Ergosterol, the major sterol found in the plasma membranes of yeast and other fungi (19). In human nutrition, ergosterol is a provitamin form of vitamin D2. Ergosterol is an important precursor in the pharmaceutical industry for the production of numerous drugs. It has a role in maintaining the integrity of the cell membrane and its fluidity. Ergosterol (ES) and ergosterol peroxide (EP) are secondary metabolites common for different mushrooms and responsible for health promoting effects, including anti-inflammatory, anticancer, antiviral activity, and reduction of the incidence of cardiovascular disease.
Definition
ChEBI: A phytosterol consisting of ergostane having double bonds at the 5,6-, 7,8- and 22,23-positions as well as a 3beta-hydroxy group.
Hazard
Due to its ability to catalyze calcium deposition in the bony structure (thus preventing rickets), overdosage of vitamin D may be harmful.
InChI:InChI=1/C28H44O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h7-10,18-20,22,24-26,29H,11-17H2,1-6H3/b8-7+/t19?,20?,22-,24+,25?,26-,27-,28+/m0/s1
57-87-4 Relevant articles
Novel Dioxolane Ring Compounds for the Management of Phytopathogen Diseases as Ergosterol Biosynthesis Inhibitors: Synthesis, Biological Activities, and Molecular Docking
Li-Jing Min, Han Wang, Joanna Bajsa-Hirschel, Chen-Sheng Yu, Bin Wang, Meng-Meng Yao, Liang Han, Charles L. Cantrell, Stephen O. Duke*, Na-Bo Sun*, and Xing-Hai Liu*
J. Agric. Food Chem. 2022, 70, 14, 4303–4315
Molecular simulation docking results of compound D26 and difenoconazole with fungal CYP51 P450 confirmed that they both inhibit this enzyme involved in ergosterol biosynthesis. The structure–activity relationships (SAR) are discussed by substituent effect, molecular docking, and density functional theory analysis, which provided useful information for designing more active compounds.
Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae
Ziqian Guo, Xinyu Liu, Nian Wang, Pengcheng Mo, Ju Shen, Muxing Liu, Haifeng Zhang, Ping Wang, Zhengguang Zhang
New Phytologist Volume237, Issue3 February 2023 Pages 930-943
Our findings suggested that ergosterol-enriched lipid rafts constitute a platform for interactions among various SNARE proteins that are required for the development and pathogenicity of M. oryzae.
57-87-4 Process route
-
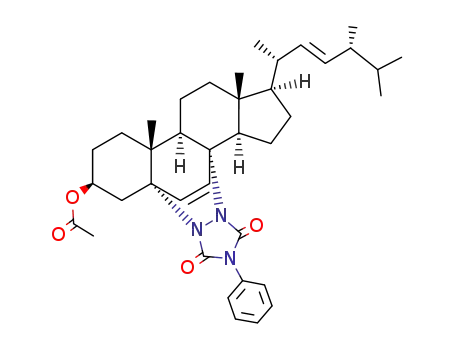
- 28421-56-9
3β-acetoxy-5α,8α-(4-phenyl-1,2,4-triazolidine-3,5-dione-1,2-diyl)ergosta-6,22-diene

-
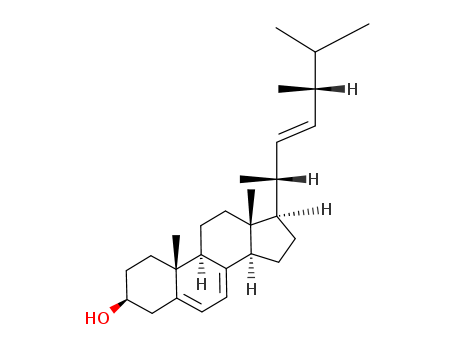
- 57-87-4
Ergosterol

-
![1-Isobutyl-4-phenyl-[1,2,4]triazolidin-3-one](/upload/2023/1/5e38b93f-a5e3-4f8a-ba30-814566274da4.png)
-
1-Isobutyl-4-phenyl-[1,2,4]triazolidin-3-one
| Conditions | Yield |
|---|---|
|
With diisobutylaluminium hydride; In hexane; toluene; at 0 ℃; for 0.166667h;
|
70% |
-
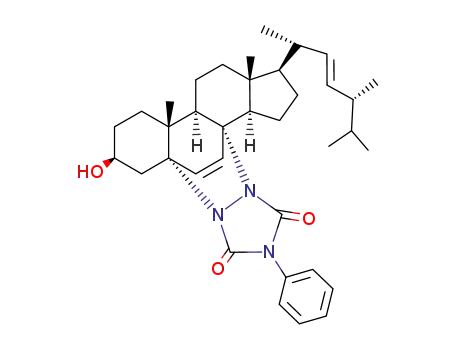
- 10123-90-7
3β-hydroxy-3',5'-dioxo-4'-phenyl-5,8<1',2'>-1',2',4'-triazolidino-5α,8α-ergosta-6,22-diene

-

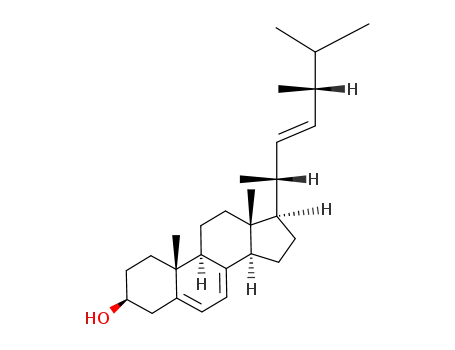
- 57-87-4
Ergosterol

-
![1-Isobutyl-4-phenyl-[1,2,4]triazolidin-3-one](/upload/2023/1/5e38b93f-a5e3-4f8a-ba30-814566274da4.png)
-
1-Isobutyl-4-phenyl-[1,2,4]triazolidin-3-one
| Conditions | Yield |
|---|---|
|
With diisobutylaluminium hydride; In hexane; toluene; at 0 ℃; for 0.166667h;
|
51% |
57-87-4 Upstream products
-
60-29-7
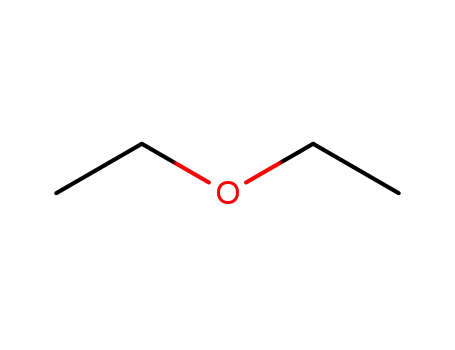
diethyl ether
-
21307-05-1
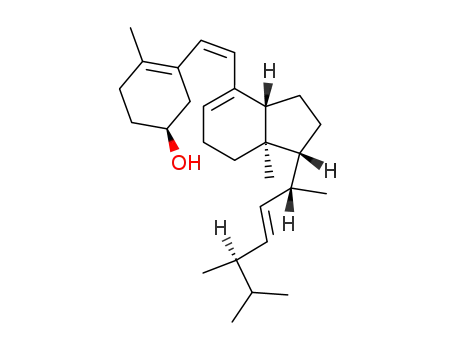
previtamin D2
-
17398-57-1
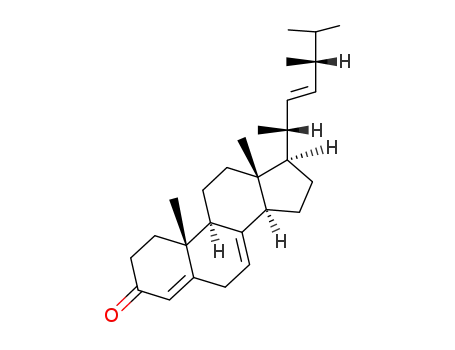
(22E,24R)-ergosta-4,7,22-triene-3-one
-
19044-12-3
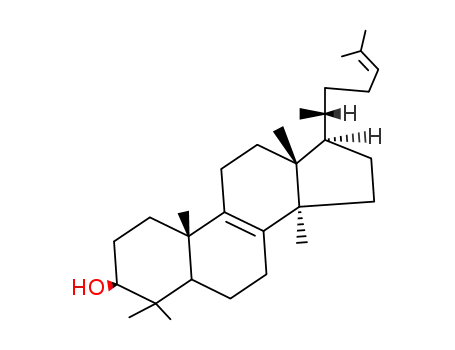
lanosterol
57-87-4 Downstream products
-
55401-50-8
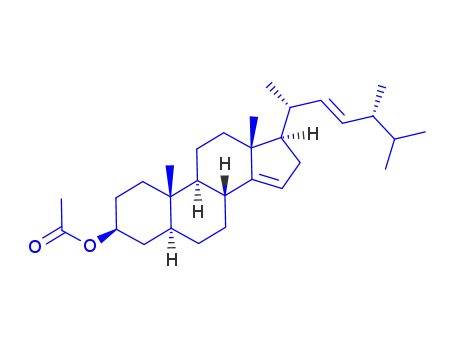
(3S,5S,8R,9S,10S,13R,17R)-17-((2R,5R,E)-5,6-dimethylhept-3-en-2-yl)-10,13-dimethyl-2,3,4,5,6,7,8,9,10,11,12,13,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate
-
705926-28-9
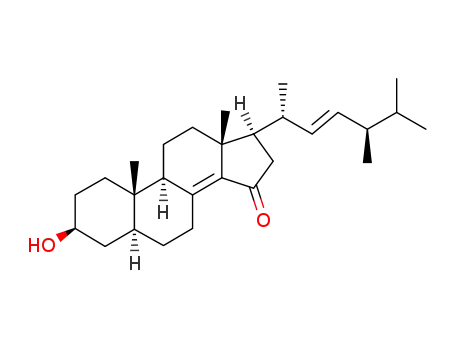
(22E)-3β-hydroxyergosta-8(14),22-dien-15-one
-
115-61-7
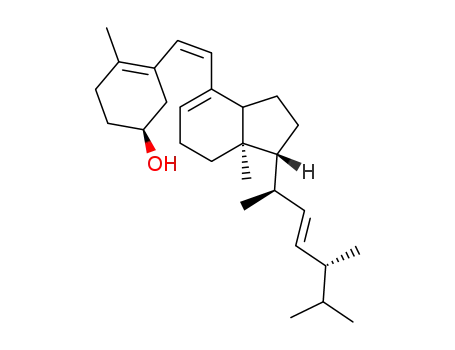
previtamin D2
-
39382-93-9
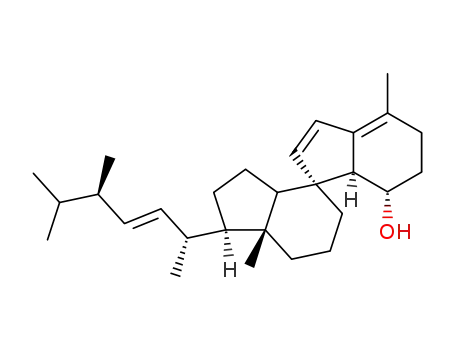
toxisterol2 A2
Relevant Products
-
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one 196597-78-1
CAS:196597-78-1
-
Benzeneethanol, b-(dimethylamino)-b-ethyl- 39068-94-5
CAS:39068-94-5
-
Mannitol
CAS:87-78-5